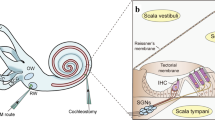
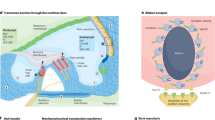
Abstract
Hearing loss is the most common sensory deficit in humans and can result from genetic, environmental or combined etiologies that prevent normal function of the cochlea, the peripheral sensory organ. Recent advances in understanding the genetic pathways that are critical for the development and maintenance of cochlear function, as well as the molecular mechanisms that underlie cell trauma and death, have provided exciting opportunities for modulating these pathways to correct genetic mutations, to enhance the endogenous protective pathways for hearing preservation and to regenerate lost sensory cells with the possibility of ameliorating hearing loss. A number of recent animal studies have used gene-based therapies in innovative ways toward realizing these goals. With further refinement, some of the protective and regenerative approaches reviewed here may become clinically applicable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Akil O, Seal RP, Burke K, Wang C, Alemi A, During M et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012; 75: 283–293.
Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 2013; 77: 58–69.
Wilson BS, Dorman MF . Cochlear implants: current designs and future possibilities. J Rehabil Res Dev 2008; 45: 695–730.
Dror AA, Avraham KB . Hearing impairment: a panoply of genes and functions. Neuron 2010; 68: 293–308.
Dror AA, Avraham KB . Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet 2009; 43: 411–437.
Dolan DF, Yamasoba T, Leonova E, Beyer LA, Raphael Y . Morphological and physiological effects of long duration infusion of strychnine into the organ of Corti. J Neurocytol 1999; 28: 197–206.
Mencía A, Modamio-Høybjør S, Redshaw N, Morín M, Mayo-Merino F, Olavarrieta L et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet 2009; 41: 609–613.
Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Vanrybroek J et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet 2008; 83: 278–292.
Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 2008; 57: 263–275.
Maeda Y, Fukushima K, Nishizaki K, Smith RJ . In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet 2005; 14: 1641–1650.
Schoen CJ, Burmeister M, Lesperance MM . Diaphanous homolog 3 (Diap3) overexpression causes progressive hearing loss and inner hair cell defects in a transgenic mouse model of human deafness. PLoS One 2013; 8: e56520.
Schoen CJ, Emery SB, Thorne MC, Ammana HR, Sliwerska E, Arnett J et al. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc Natl Acad Sci USA 2010; 107: 13396–13401.
Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J et al. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther 2004; 9: 173–181.
Yagi M, Magal E, Sheng Z, Ang KA, Raphael Y . Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor. Hum Gene Ther 1999; 10: 813–823.
Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y . Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther 2003; 7: 484–492.
Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther 2010; 18: 1111–1122.
Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD . Neurotrophin-3 transduction attenuates Cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther 2002; 6: 12–18.
Pfannenstiel SC, Praetorius M, Plinkert PK, Brough DE, Staecker H . Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol Neurootol 2009; 14: 254–266.
Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP . Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal 2010; 13: 589–598.
Du X, Chen K, Kuriyavar S, Kopke RD, Grady BP, Bourne DH et al. Magnetic targeted delivery of dexamethasone acetate across the round window membrane in guinea pigs. Otol Neurotol 2013; 34: 41–47.
Mikuriya T, Sugahara K, Takemoto T, Tanaka K, Takeno K, Shimogori H et al. Geranylgeranylacetone, a heat shock protein inducer, prevents acoustic injury in the guinea pig. Brain Res 2005; 1065: 107–114.
Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL et al. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res 2006; 218: 20–29.
Raphael Y, Altschuler RA . Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton 1991; 18: 215–227.
Forge A . Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res 1985; 19: 171–182.
Chen P, Johnson JE, Zoghbi HY, Segil N . The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 2002; 129: 2495–2505.
Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y . Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 2003; 23: 4395–4400.
Staecker H, Praetorius M, Baker K, Brough DE . Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol 2007; 28: 223–231.
Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y . Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res 2008; 240: 52–56.
Wu DK, Kelley MW . Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol 2012; 4: a008409.
Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J . Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport 2007; 18: 1911–1914.
Minoda R, Izumikawa M, Kawamoto K, Zhang H, Raphael Y . Manipulating cell cycle regulation in the mature cochlea. Hear Res 2007; 232: 44–51.
Kanzaki S, Beyer LA, Swiderski DL, Izumikawa M, Stover T, Kawamoto K et al. p27(Kip1) deficiency causes organ of Corti pathology and hearing loss. Hear Res 2006; 214: 28–36.
Chen P, Segil N . p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 1999; 126: 1581–1590.
Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci USA 1999; 96: 4084–4088.
Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 2005; 307: 1114–1118.
Fukui H, Wong HT, Beyer LA, Case BG, Swiderski DL, Di Polo A et al. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep 2012; 2: 838.
Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE et al. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol 2010; 223: 464–472.
Ohlemiller KK . Mechanisms and genes in human strial presbycusis from animal models. Brain Res 2009; 1277: 70–83.
Spicer SS, Schulte BA . Pathologic changes of presbycusis begin in secondary processes and spread to primary processes of strial marginal cells. Hear Res 2005; 205: 225–240.
Husseman J, Raphael Y . Gene therapy in the inner ear using adenovirus vectors. Adv Otorhinolaryngol 2009; 66: 37–51.
Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, Bennett J . In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther 2006; 14: 328–335.
Iizuka T, Kanzaki S, Mochizuki H, Inoshita A, Narui Y, Furukawa M et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther 2008; 19: 384–390.
Lalwani AK, Mhatre AN . Cochlear gene therapy. Ear Hear 2003; 24: 342–348.
Wenzel GI, Xia A, Funk E, Evans MB, Palmer DJ, Ng P et al. Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otol Neurotol 2007; 28: 1100–1108.
Shibata SB, Di Pasquale G, Cortez SR, Chiorini JA, Raphael Y . Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther 2009; 16: 990–997.
Derby ML, Sena-Esteves M, Breakefield XO, Corey DP . Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res 1999; 134: 1–8.
Chen X, Frisina RD, Bowers WJ, Frisina DR, Federoff HJ . HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol Ther 2001; 3: 958–963.
Maeda Y, Fukushima K, Nishizaki K, Smith RJ . In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet 2005; 14: 1641–1650.
Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y . siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol Ther 2013; 21: 834–841.
Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope 2005; 115: 2000–2005.
Ge X, Jackson RL, Liu J, Harper EA, Hoffer ME, Wassel RA et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg 2007; 137: 619–623.
Buckiova D, Ranjan S, Newman TA, Johnston AH, Sood R, Kinnunen PK et al. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine (Lond) 2012; 7: 1339–1354.
Yogalingam G, Hopwood JJ . Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: diagnostic, clinical, and biological implications. Hum Mutat 2001; 18: 264–281.
Heldermon CD, Qin EY, Ohlemiller KK, Herzog ED, Brown JR, Vogler C et al. Disease correction by combined neonatal intracranial AAV and systemic lentiviral gene therapy in Sanfilippo Syndrome type B mice. Gene Ther 2013, e-pub ahead of print 28 March 2013; doi:10.1038/gt.2013.14.
Fukui H, Raphael Y . Gene therapy for the inner ear. Hear Res 2012; 297: 99–105.
Sacheli R, Delacroix L, Vandenackerveken P, Nguyen L, Malgrange B . Gene transfer in inner ear cells: a challenging race. Gene Ther 2013; 20: 237–247.
Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC et al. Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in pendred syndrome. J Assoc Res Otolaryngol 2003; 4: 394–404.
Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2004; 2: 30.
Lautermann J, Frank HG, Jahnke K, Traub O, Winterhager E . Developmental expression patterns of connexin26 and −30 in the rat cochlea. Dev Genet 1999; 25: 306–311.
Kikuchi T, Kimura RS, Paul DL, Adams JC . Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 1995; 191: 101–118.
Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H et al. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 1998; 294: 415–420.
Wise AK, Richardson R, Hardman J, Clark G, O'Leary S . Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol 2005; 487: 147–165.
Acknowledgements
We would like to thank Hiu Tung (Candy) Wong for producing the schematic figure. Our work is funded by the R Jamison and Betty Williams Professorship, the Hirschfield Foundation, MedEl and the NIH/NIDCD Grants: R01-DC010412, R01-DC007634 and P30-DC05188.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kohrman, D., Raphael, Y. Gene therapy for deafness. Gene Ther 20, 1119–1123 (2013). https://doi.org/10.1038/gt.2013.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.39
Keywords
This article is cited by
-
In vivo genetic manipulation of inner ear connexin expression by bovine adeno-associated viral vectors
Scientific Reports (2017)
-
Epithelial–mesenchymal transition, and collective and individual cell migration regulate epithelial changes in the amikacin-damaged organ of Corti
Histochemistry and Cell Biology (2017)
-
Emerging Gene Therapies for Genetic Hearing Loss
Journal of the Association for Research in Otolaryngology (2017)