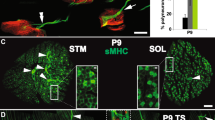
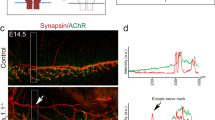
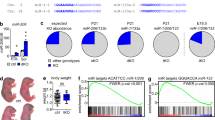
Abstract
Motor axons approach muscles that are regionally prespecialized, as acetylcholine receptors are clustered in the central region of muscle before and independently of innervation. This muscle prepattern requires MuSK, a receptor tyrosine kinase that is essential for synapse formation. It is not known how muscle prepatterning is established, and whether motor axons recognize this prepattern. Here we show that expression of Musk is prepatterned in muscle and that early Musk expression in developing myotubes is sufficient to establish muscle prepatterning. We further show that ectopic Musk expression promotes ectopic synapse formation, indicating that muscle prepatterning normally has an instructive role in directing where synapses will form. In addition, ectopic Musk expression stimulates synapse formation in the absence of Agrin and rescues the lethality of Agrn mutant mice, demonstrating that the postsynaptic cell, and MuSK in particular, has a potent role in regulating the formation of synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
11 January 2008
In the version of this article initially published online, several items were omitted from the text. On page 20, left column, the sentence “To determine whether this restricted pattern…regulatory region of the human skeletal α-actin (HAS; Fig. 1e)” should read “To determine whether this restricted pattern…regulatory region of the human skeletal α-actin gene (HAS; Fig. 1e)”. On page 25, left column, the sentence “Muscle is pre-specialized in the central, prospective synaptic region before and independently of innervation, and have led to a revised model of the steps and mechanisms that regulate neuromuscular synapse formation” should read “Muscle is pre-specialized in the central, prospective synaptic region before and independently of innervation, and these findings have led to a revised model of the steps and mechanisms that regulate neuromuscular synapse formation.” Finally, on page 19, left column, the sentence “This organizational feature of neurons is essential for forming synapses on appropriate target cells and establishing functional neuronal circuits” should read “This organizational feature of neurons is critical for forming synapses on appropriate target cells and establishing functional neuronal circuits.” These errors have been corrected in the HTML and PDF versions of the article.
References
Burden, S.J. The formation of neuromuscular synapses. Genes Dev. 12, 133–148 (1998).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Sanes, J.R. & Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 (2001).
Bennett, M.R. & Pettigrew, A.G. The formation of synapses in striated muscle during development. J. Physiol. (Lond.) 241, 515–545 (1974).
Bennett, M.R. & Pettigrew, A.G. The formation of neuromuscular synapses. Cold Spring Harb. Symp. Quant. Biol. 40, 409–424 (1976).
Kandel, E.R., Schwartz, J.H. & Jessell, T.M. Principles of Neural Science (McGraw-Hill, Health Professions Division, New York, 2000).
Schaeffer, L., de Kerchove d' Exaerde, A. & Changeux, J.P. Targeting transcription to the neuromuscular synapse. Neuron 31, 15–22 (2001).
Valenzuela, D.M. et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15, 573–584 (1995).
Glass, D.J. et al. Agrin acts via a MuSK receptor complex. Cell 85, 513–523 (1996).
Glass, D.J. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation and is a functional receptor for agrin. Cold Spring Harb. Symp. Quant. Biol. 61, 435–444 (1996).
Glass, D.J. & Yancopoulos, G.D. Sequential roles of agrin, MuSK and rapsyn during neuromuscular junction formation. Curr. Opin. Neurobiol. 7, 379–384 (1997).
Yang, X., Li, W., Prescott, E.D., Burden, S.J. & Wang, J.C. DNA topoisomerase IIβ and neural development. Science 287, 131–134 (2000).
Lin, W. et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064 (2001).
Yang, X. et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399–410 (2001).
Lin, W. et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 46, 569–579 (2005).
Misgeld, T., Kummer, T.T., Lichtman, J.W. & Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 102, 11088–11093 (2005).
Flanagan-Steet, H., Fox, M.A., Meyer, D. & Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 132, 4471–4481 (2005).
Panzer, J.A., Song, Y. & Balice-Gordon, R.J. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J. Neurosci. 26, 934–947 (2006).
DeChiara, T.M. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 501–512 (1996).
Strochlic, L., Cartaud, A. & Cartaud, J. The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions. BioEssays 27, 1129–1135 (2005).
Slater, C.R. Neural influence on the postnatal changes in acetylcholine receptor distribution at nerve-muscle junctions in the mouse. Dev. Biol. 94, 23–30 (1982).
Slater, C.R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev. Biol. 94, 11–22 (1982).
Nguyen, Q.T. & Lichtman, J.W. Mechanism of synapse disassembly at the developing neuromuscular junction. Curr. Opin. Neurobiol. 6, 104–112 (1996).
Williams, P.E. & Goldspink, G. Longitudinal growth of striated muscle fibres. J. Cell Sci. 9, 751–767 (1971).
Zhang, M. & McLennan, I.S. During secondary myotube formation, primary myotubes preferentially absorb new nuclei at their ends. Dev. Dyn. 204, 168–177 (1995).
Jones, G., Moore, C., Hashemolhosseini, S. & Brenner, H.R. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci. 19, 3376–3383 (1999).
Moore, C., Leu, M., Muller, U. & Brenner, H.R. Induction of multiple signaling loops by MuSK during neuromuscular synapse formation. Proc. Natl. Acad. Sci. USA 98, 14655–14660 (2001).
Sander, A., Hesser, B.A. & Witzemann, V. MuSK induces in vivo acetylcholine receptor clusters in a ligand-independent manner. J. Cell Biol. 155, 1287–1296 (2001).
Lacazette, E., Le Calvez, S., Gajendran, N. & Brenner, H.R. A novel pathway for MuSK to induce key genes in neuromuscular synapse formation. J. Cell Biol. 161, 727–736 (2003).
Arber, S., Burden, S.J. & Harris, A.J. Patterning of skeletal muscle. Curr. Opin. Neurobiol. 12, 100–103 (2002).
Minty, A.J., Alonso, S., Caravatti, M. & Buckingham, M.E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell 30, 185–192 (1982).
Brennan, K.J. & Hardeman, E.C. Quantitative analysis of the human α-skeletal actin gene in transgenic mice. J. Biol. Chem. 268, 719–725 (1993).
Fontaine, B., Sassoon, D., Buckingham, M. & Changeux, J.P. Detection of the nicotinic acetylcholine receptor α-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 7, 603–609 (1988).
Gautam, M. et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 (1996).
Hesser, B.A., Sander, A. & Witzemann, V. Identification and characterization of a novel splice variant of MuSK. FEBS Lett. 442, 133–137 (1999).
Fu, A.K., Cheung, J., Smith, F.D., Ip, F.C. & Ip, N.Y. Overexpression of muscle specific kinase increases the transcription and aggregation of acetylcholine receptors in Xenopus embryos. Brain Res. Mol. Brain Res. 96, 21–29 (2001).
Misgeld, T. et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron 36, 635–648 (2002).
Brandon, E.P. et al. Aberrant patterning of neuromuscular synapses in choline acetyltransferase–deficient mice. J. Neurosci. 23, 539–549 (2003).
Watty, A. et al. The in vitro and in vivo phosphotyrosine map of activated MuSK. Proc. Natl. Acad. Sci. USA 97, 4585–4590 (2000).
Campagna, J.A., Ruegg, M.A. & Bixby, J.L. Agrin is a differentiation-inducing 'stop signal' for motoneurons in vitro. Neuron 15, 1365–1374 (1995).
Dimitropoulou, A. & Bixby, J.L. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Mol. Cell. Neurosci. 28, 292–302 (2005).
Herbst, R., Avetisova, E. & Burden, S.J. Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development 129, 5449–5460 (2002).
Jaworski, A. & Burden, S.J. Neuromuscular synapse formation in mice lacking motor neuron– and skeletal muscle–derived Neuregulin-1. J. Neurosci. 26, 655–661 (2006).
Shefer, G. & Yablonka-Reuveni, Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 290, 281–304 (2005).
Acknowledgements
We thank T. Jessell for antibodies to Isl1/Isl2, and HB9cre and Isl2DTA mice, J. Sanes for AgrnΔZ and Agrn-null mice, E. Hardeman for pHSA2000CAT, J. Fan for technical assistance, and M. Raff, J. Dasen, W. Gan, R. Lehmann and D. Littman for comments on the manuscript. This work was supported with funds from the US National Institutes of Health (NS36193) and the Robert Packard Center for ALS Research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Methods (PDF 4473 kb)
Rights and permissions
About this article
Cite this article
Kim, N., Burden, S. MuSK controls where motor axons grow and form synapses. Nat Neurosci 11, 19–27 (2008). https://doi.org/10.1038/nn2026
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2026
This article is cited by
-
LRP4 is required for the olfactory association task in the piriform cortex
Cell & Bioscience (2022)
-
Bioengineered model of the human motor unit with physiologically functional neuromuscular junctions
Scientific Reports (2021)
-
Mechanism of disease and therapeutic rescue of Dok7 congenital myasthenia
Nature (2021)
-
Heterogeneous Nuclear Ribonucleoproteins: Implications in Neurological Diseases
Molecular Neurobiology (2021)
-
Centrosome and ciliary abnormalities in fetal akinesia deformation sequence human fibroblasts
Scientific Reports (2020)