Abstract
Background
While environmental factors play an important role in weight loss effectiveness, genetics may also influence its success. We examined whether a genome-wide polygenic score for BMI was associated with weight loss effectiveness and aimed to identify common genetic variants associated with weight loss.
Methods
Participants in the ONTIME study (n = 1210) followed a uniform, multimodal behavioral weight-loss intervention. We first tested associations between a genome-wide polygenic score for higher BMI and weight loss effectiveness (total weight loss, rate of weight loss, and attrition). We then conducted a genome-wide association study (GWAS) for weight loss in the ONTIME study and performed the largest weight loss meta-analysis with earlier studies (n = 3056). Lastly, we ran exploratory GWAS in the ONTIME study for other weight loss outcomes and related factors.
Results
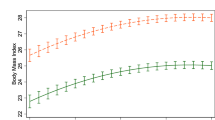
We found that each standard deviation increment in the polygenic score was associated with a decrease in the rate of weight loss (Beta (95% CI) = −0.04 kg per week (−0.06, −0.01); P = 3.7 × 10−03) and with higher attrition after adjusting by treatment duration. No associations reached genome-wide significance in meta-analysis with previous GWAS studies for weight loss. However, associations in the ONTIME study showed effects consistent with published studies for rs545936 (MIR486/NKX6.3/ANK1), a previously noted weight loss locus. In the meta-analysis, each copy of the minor A allele was associated with 0.12 (0.03) kg/m2 higher BMI at week five of treatment (P = 3.9 × 10−06). In the ONTIME study, we also identified two genome-wide significant (P < 5×10−08) loci for the rate of weight loss near genes implicated in lipolysis, body weight, and metabolic regulation: rs146905606 near NFIP1/SPRY4/FGF1; and rs151313458 near LSAMP.
Conclusion
Our findings are expected to help in developing personalized weight loss approaches based on genetics.
Clinical trial registration
Obesity, Nutrigenetics, Timing, and Mediterranean (ONTIME; clinicaltrials.gov: NCT02829619) study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Summary statistics from this genome-wide association study can be downloaded from the Common Metabolic Diseases Knowledge Portal (https://hugeamp.org/).
Change history
08 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41366-024-01497-4
References
Chopra S, Malhotra A, Ranjan P, Vikram NK, Sarkar S, Siddhu A, et al. Predictors of successful weight loss outcomes amongst individuals with obesity undergoing lifestyle interventions: a systematic review. Obes Rev. 2021; 22. https://doi.org/10.1111/OBR.13148.
Dashti HS, Gómez-Abellán P, Qian J, Esteban A, Morales E, Scheer FAJL, et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2021;113:154–61.
Hatoum IJ, Greenawalt DM, Cotsapas C, Reitman ML, Daly MJ, Kaplan LM. Heritability of the weight loss response to gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E1630–E1633.
Lopez-Minguez J, Dashti HS, Madrid-Valero JJ, Madrid JA, Saxena R, Scheer FAJL, et al. Heritability of the timing of food intake. Clin Nutr. 2018. https://doi.org/10.1016/j.clnu.2018.03.002.
Loos RJF, Yeo GSH. The bigger picture of FTO - The first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61.
Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. 2012;3:29.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Dashti HS, Levy DE, Hivert MF, Alimenti K, McCurley JL, Saxena R, et al. Genetic risk for obesity and the effectiveness of the ChooseWell 365 workplace intervention to prevent weight gain and improve dietary choices. Am J Clin Nutr. 2022;115:180–8.
Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177:587–596.e9.
Ding M, Ellervik C, Huang T, Jensen MK, Curhan GC, Pasquale LR, et al. Diet quality and genetic association with body mass index: results from 3 observational studies. Am J Clin Nutr. 2018;108:1291–1300.
Moon J-Y, Wang T, Sofer T, North KE, Isasi CR, Cai J, et al. Objectively measured physical activity, sedentary behavior, and genetic predisposition to obesity in U.S. Hispanics/Latinos: results from the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes. 2017;66:3001–12.
Robino A, Concas MP, Catamo E, Gasparini P. A brief review of genetic approaches to the study of food preferences: current knowledge and future directions. Nutrients. 2019; 11. https://doi.org/10.3390/nu11081735.
Ranzenhofer LM, Mayer LES, Davis HA, Mielke‐Maday HK, McInerney H, Korn R, et al. The FTO gene and measured food intake in 5‐ to 10‐year‐old children without obesity. Obesity. 2019;27:1023–9.
van der Klaauw AA, Keogh JM, Henning E, Stephenson C, Kelway S, Trowse VM, et al. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat Commun. 2016;7:13055.
Lamiquiz-Moneo I, Mateo-Gallego R, Bea AM, Dehesa-García B, Pérez-Calahorra S, Marco-Benedí V, et al. Genetic predictors of weight loss in overweight and obese subjects. Sci Rep. 2019; 9. https://doi.org/10.1038/S41598-019-47283-5.
Heitkamp M, Siegrist M, Molnos S, Brandmaier S, Wahl S, Langhof H, et al. Obesity genes and weight loss during lifestyle intervention in children with obesity. JAMA Pediatr. 2021;175:e205142. https://doi.org/10.1001/jamapediatrics.2020.5142.
Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2021;23:120–33.
Valsesia A, Wang QP, Gheldof N, Carayol J, Ruffieux H, Clark T, et al. Genome-wide gene-based analyses of weight loss interventions identify a potential role for NKX6.3 in metabolism. Nature Commun. 2019;10:1–10.
Papandonatos GD, Pan Q, Pajewski NM, Delahanty LM, Peter I, Erar B, et al. Genetic predisposition to weight loss and regain with lifestyle intervention: analyses from the diabetes prevention program and the look AHEAD randomized controlled trials. Diabetes. 2015;64:4312–21.
Corbalán MD, Morales EM, Canteras M, Espallardo A, Hernández T, Garaulet M. Effectiveness of cognitive-behavioral therapy based on the Mediterranean diet for the treatment of obesity. Nutrition. 2009;25:861–9.
Morales E, Torres-Castillo N, Garaulet M. Infancy and childhood obesity grade predicts weight loss in adulthood: the ontime study. Nutrients. 2021; 13. https://doi.org/10.3390/NU13072132/S1.
NHLBI Obesity education initiative expert panel on the identification E and T of O in A (US). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. 1998.
Serra-Majem L, Aranceta J. Nutritional objectives for the Spanish population. Consensus from the Spanish Society of Community Nutrition. Public Health Nutr. 2001; 4. https://doi.org/10.1079/PHN2001229.
Garaulet M, Corbalán-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, et al. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110:917–21.
Garaulet M, Canteras M, Morales E, López-Guimera G, Sánchez-Carracedo D, Corbalán-Tutau MD. Validation of a questionnaire on emotional eating for use in cases of obesity: the Emotional Eater Questionnaire (EEQ). Nutricion hospitalaria. 2012;27:645–51.
Wang C, Zhan X, Liang L, Abecasis GR, Lin X. Improved ancestry estimation for both genotyping and sequencing data using projection Procrustes analysis and genotype imputation. Am J Human Genet. 2015;96:926–37.
Cann HM, de Toma C, Cazes L, Legrand MF, Morel V, Piouffre L, et al. A human genome diversity cell line panel. Science. 2002;296:261–2.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Human Genet. 2007;81:559–75.
Ge T, Chen CY, Ni Y, Feng YCA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1–10.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–9.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1.
Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium N. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Bojarczuk A, Boulygina EA, Dzitkowska-Zabielska M, Łubkowska B, Leońska-Duniec A, Egorova ES, et al. Genome-wide association study of exercise-induced fat loss efficiency. Genes. 2022; 13. https://doi.org/10.3390/GENES13111975.
McCaffery JM, Jablonski KA, Pan Q, Astrup A, Revsbech Christiansen M, Corella D, et al. Genetic predictors of change in waist circumference and waist-to-hip ratio with lifestyle intervention: the trans-NIH consortium for genetics of weight loss response to lifestyle intervention. Diabetes. 2022;71:669–76.
Appel LJ, Clark JM, Yeh H-C, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–68.
Wing RR, Crane MM, Thomas JG, Kumar R, Weinberg B. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health. 2010;100:2513–9.
Asbjørnsen RA, Smedsrød ML, Nes LS, Wentzel J, Varsi C, Hjelmesæth J, et al. Persuasive system design principles and behavior change techniques to stimulate motivation and adherence in electronic health interventions to support weight loss maintenance: scoping review. J Med Internet Res. 2019; 21. https://doi.org/10.2196/14265.
Metabolic Disorders Knowledge Portal - Home. https://hugeamp.org/. 2022.
Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, et al. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–4.
Sancar G, Liu S, Gasser E, Alvarez JG, Moutos C, Kim K, et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab. 2022;34:171–183.e6.
Wang A, Yan X, Zhang C, Du C, Long W, Zhan D, et al. Characterization of fibroblast growth factor 1 in obese children and adolescents. Endocr Connect. 2018;7:932–40.
Hasegawa S, Ikeda Y, Yamasaki M, Fukui T. The role of acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, in 3T3-L1 adipocyte differentiation. Biol Pharm Bull. 2012;35:1980–5.
Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, et al. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–20.
Zondag GCM, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn). J Biol Chem. 2000;275:11264–9.
Funding
The ONTIME study received the following funding: PID2020-112768RB-I00 funded by MCIN/AEI/10.13039/501100011033. The Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18) and NIDDK R01DK105072 granted to MG. RS was supported by NIH R01DK107859 and Phyllis and Jerome Lyle Rappaport Massachusetts General Hospital Research Scholar Award. RS and FAJLS were supported by NIH R01DK102696 and R01DK105072. FAJLS was further supported by R01DK099512, R01HL118601, R01HL140574, and R01HL153969. HSD is supported by NIH K99HL153795.
Author information
Authors and Affiliations
Contributions
All authors listed fully meet the criteria for authorship. The authors’ responsibilities were as follows: Conceptualization, HSD, RS, and MG; methodology, HSD, FAJLS, RS, and MG; formal analysis, HSD and MG; resources HSD and MG; data curation MG; writing— original draft preparation, HSD and MG; writing—review and editing, HSD, FAJLS, RS, and MG; visualization, HSD and MG; supervision, RS and MG. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
FAJLS served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham and Morehouse School of Medicine. FAJLS interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. FAJLS consultancies are not related to the current work. RS is a cofounder of Magnet Biomedicine, not related to the current work. The other authors declare no conflict of interest. All authors have read the Journal’s policy on disclosure of potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dashti, H.S., Scheer, F.A.J.L., Saxena, R. et al. Impact of polygenic score for BMI on weight loss effectiveness and genome-wide association analysis. Int J Obes 48, 694–701 (2024). https://doi.org/10.1038/s41366-024-01470-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-024-01470-1