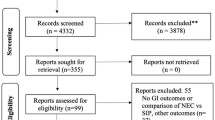
Abstract
Objective
To determine if intestinal perforations before 14 days (either spontaneous (SIP) or necrotizing enterocolitis-induced) are increased when infants who received antenatal betamethasone shortly before birth are treated with prophylactic indomethacin (PINDO).
Study design
Observational study of 475 infants <28 week’s gestation assigned to either a PINDO-protocol (n = 231) or expectant management protocol (n = 244) during consecutive protocol epochs.
Results
Intestinal perforations before 14 days occurred in 33/475 (7%). In unadjusted and adjusted models, we found no associations between PINDO-protocol and intestinal perforations. PINDO-protocol did not increase intestinal perforations or SIP-alone even when given to infants who received betamethasone <7 or <2 days before delivery. 213/231 (92%) PINDO-protocol infants actually received indomethacin. The results were unchanged when examined just in those who received indomethacin.
Conclusion
In our study, early intestinal perforations and SIP-alone were not increased when PINDO was used by protocol in infants who received antenatal betamethasone shortly before birth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shah J, Singhal N, da Silva O, Rouvinez-Bouali N, Seshia M, Lee SK, et al. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol. 2015;35:595–600.
Ang JL, Rath CP, Tan H, Patole S, Rao SC. Mortality and neurodevelopmental outcomes of infants with spontaneous intestinal perforation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2022. https://doi.org/10.1136/archdischild-2022-324157
Najaf TA, Vachharajani NA, Warner BW, Vachharajani AJ. Interval between clinical presentation of necrotizing enterocolitis and bowel perforation in neonates. Pediatr Surg Int. 2010;26:607–9.
Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS One. 2012;7:e36977.
Caplan MS, Fanaroff A. Necrotizing: a historical perspective. Semin Perinatol. 2017;41:2–6.
Hackam D, Caplan M. Necrotizing enterocolitis: pathophysiology from a historical context. Semin Pediatr Surg. 2018;27:11–8.
Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? J Perinatol. 2007;27:661–71.
Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–44.
Pumberger W, Mayr M, Kohlhauser C, Weninger M. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. 2002;195:796–803.
Lai S, Yu W, Wallace L, Sigalet D. Intestinal muscularis propria increases in thickness with corrected gestational age and is focally attenuated in patients with isolated intestinal perforations. J Pediatr Surg. 2014;49:114–9.
Ragouilliaux CJ, Keeney SE, Hawkins HK, Rowen JL. Maternal factors in extremely low birth weight infants who develop spontaneous intestinal perforation. Pediatrics. 2007;120:e1458–64.
Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. 2006;26:93–9.
Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl J Med. 2001;344:95–101.
Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004;114:1649–57.
Paquette L, Friedlich P, Ramanathan R, Seri I. Concurrent use of indomethacin and dexamethasone increases the risk of spontaneous intestinal perforation in very low birth weight neonates. J Perinatol. 2006;26:486–92.
Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA. Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. 2016;175:775–83.
Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;2010:CD000174.
Gordon PV, Herman AC, Marcinkiewicz M, Gaston BM, Laubach VE, Aschner JL. A neonatal mouse model of intestinal perforation: investigating the harmful synergism between glucocorticoids and indomethacin. J Pediatr Gastroenterol Nutr. 2007;45:509–19.
Sharma R, Hudak ML, Tepas JJ 3rd, Wludyka PS, Teng RJ, Hastings LK, et al. Prenatal or postnatal indomethacin exposure and neonatal gut injury associated with isolated intestinal perforation and necrotizing enterocolitis. J Perinatol. 2010;30:786–93.
Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J Perinatol. 2017;37:188–93.
Qureshi M, Shah PS, Abdelgadir D, Ye XY, Afifi J, Yuen R, et al. Gestational age-dependent variations in effects of prophylactic indomethacin on brain injury and intestinal injury. J Pediatr. 2021;235:26–33.
Chawla S, Natarajan G, Laptook AR, Chowdhury D, Bell EF, Ambalavanan N, et al. Model for severe intracranial hemorrhage and role of early indomethacin in extreme preterm infants. Pediatr Res. 2022;92:1648–56.
Shorter NA, Liu JY, Mooney DP, Harmon BJ. Indomethacin-associated bowel perforations: a study of possible risk factors. J Pediatr Surg. 1999;34:442–4.
Wadhawan R, Oh W, Vohr BR, Saha S, Das A, Bell EF, et al. Spontaneous intestinal perforation in extremely low birth weight infants: association with indometacin therapy and effects on neurodevelopmental outcomes at 18–22 months corrected age. Arch Dis Child Fetal Neonatal Ed. 2013;98:F127–32.
Kelleher J, Salas AA, Bhat R, Ambalavanan N, Saha S, Stoll BJ, et al. Prophylactic indomethacin and intestinal perforation in extremely low birth weight infants. Pediatrics. 2014;134:e1369–77.
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl J Med. 2001;344:1966–72.
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–50.
Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99:F99–104.
de Waal K, Phad N, Stubbs M, Chen Y, Kluckow M. A randomized placebo-controlled pilot trial of early targeted nonsteroidal anti-inflammatory drugs in preterm infants with a patent ductus arteriosus. J Pediatr. 2021;228:82–6.
Attridge JT, Clark R, Gordon PV. New insights into spontaneous intestinal perforation using a national data set (3): antenatal steroids have no adverse association with spontaneous intestinal perforation. J Perinatol. 2006;26:667–70.
Kajantie E, Raivio T, Janne OA, Hovi P, Dunkel L, Andersson S. Circulating glucocorticoid bioactivity in the preterm newborn after antenatal betamethasone treatment. J Clin Endocrinol Metab. 2004;89:3999–4003.
Arnautovic TI, Longo JL, Trail-Burns EJ, Tucker R, Keszler M, Laptook AR. Antenatal risk factors associated with spontaneous intestinal perforation in preterm infants receiving postnatal indomethacin. J Pediatr. 2021;232:59–64.
Kandraju H, Kanungo J, Lee KS, Daspal S, Adie MA, Dorling J, et al. Association of co-exposure of antenatal steroid and prophylactic indomethacin with spontaneous intestinal perforation. J Pediatr. 2021;235:34–41.
Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–21.
Liebowitz M, Clyman RI. Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: effects on neonatal outcomes. J Pediatr. 2017;187:119–26.
Clyman RI, Wickremasinghe A, Merritt TA, Solomon T, McNamara P, Jain A, et al. Hypotension following patent ductus arteriosus ligation: the role of adrenal hormones. J Pediatr. 2014;164:1449–55.
Liebowitz MC, Clyman RI. Predicting the need for home oxygen therapy in preterm infants born before 28 weeks’ gestation. Am J Perinatol. 2016;33:34–9.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–7.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight <1500 grams. J Pediatr. 1978;92:529–34.
Blakely ML, Tyson JE, Lally KP, Hintz SR, Eggleston B, Stevenson DK, et al. Initial laparotomy versus peritoneal drainage in extremely low birthweight infants with surgical necrotizing enterocolitis or isolated intestinal perforation: a multicenter randomized clinical trial. Ann Surg. 2021;274:e370–e80.
Clyman RI, Jin C, Hills NK. A role for neonatal bacteremia in deaths due to intestinal perforation: spontaneous intestinal perforation compared with perforated necrotizing enterocolitis. J Perinatol. 2020;40:1662–70.
Caplan MS, Underwood MA, Modi N, Patel R, Gordon PV, Sylvester KG, et al. Necrotizing enterocolitis: using regulatory science and drug development to improve outcomes. J Pediatr. 2019;212:208–15.
Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75.
Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, Watterberg KL. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr. 2019;207:136–42.
Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF, et al. A three-day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomized trial. Pediatrics 1999;104:91–9.
Vermont Oxford Network Steroid Study G. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics. 2001;108:741–8.
Renolleau C, Toumazi A, Bourmaud A, Benoist JF, Chevenne D, Mohamed D, et al. Association between baseline cortisol serum concentrations and the effect of prophylactic hydrocortisone in extremely preterm infants. J Pediatr. 2021;234:65–70.
McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12:CD004454.
Acknowledgements
We would like to thank the neonatal and pediatric cardiology faculty, fellows, nurse practitioners, nurses, respiratory therapists and dieticians for their excellent care and their commitment to the nursery’s quality improvement projects and its consensus driven protocols.
Funding
This work was supported by a grant from U.S. Public Health Service NHLBI (HL109199) and by gifts from the Clyman Family Foundation. This work was supported by a grant from the U.S. Public Health Service National Heart, Lung, and Blood Institute (HL109199).
Author information
Authors and Affiliations
Contributions
RC was the principal investigator of the overall study and was involved with the conceptualization and design of the study, study oversight, funding acquisition, data acquisition and formal analysis, writing of the first draft in addition to performing all 4 of the following tasks: (1) participated in the design of the study, acquired patient data, and submitted IRB proposal. (2) reviewed, revised and edited the manuscript. (3) gave approval to the final version of the manuscript. (4) agreed to be accountable for all aspects of the work. NKH performed the statistical consultation and analyses in addition to performing all 4 of the following tasks: (1) participated in the design of the original protocol and performed statistical analyses. (2) reviewed, revised and edited the manuscript. (3) gave approval to the final version of the manuscript. (4) agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
We have no conflict of interests. Neither of the authors has any potential conflict of interest, real or perceived; Neither of the authors has any financial agreement with any company whose product figures prominently in the manuscript. There are no “sponsors” of this project. And there are no “sponsors” who have had a role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication. Dr. Clyman wrote the first draft of the manuscript and no honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clyman, R.I., Hills, N.K. Prophylactic indomethacin, antenatal betamethasone, and the risk of intestinal perforation in infants <28 weeks’ gestation. J Perinatol 43, 1252–1261 (2023). https://doi.org/10.1038/s41372-023-01653-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01653-0