Abstract
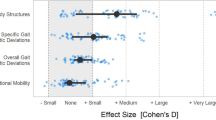
The aim of this scoping review is to examine the extent and depth of the literature on effects of central nervous system (CNS) stimulant medications on physical function in children with cerebral palsy (CP). A systematic search for relevant peer-reviewed studies was conducted of PubMed, CINAHL, Cochrane, SPORTDiscus, Embase, & Scopus (January 2002 & August 2022). We included studies that examined the effects of CNS stimulants on physical function in children with CP. Four studies met our selection criteria. All studies explored the effect of Modafinil on physical function outcomes. Three studies of the four included studies reported positive effects of Modafinil on spasticity, motor performance, and gait, whereas one study reported no significant effects of Modafinil. Our findings suggest that there is very low-quality evidence that suggests that Modafinil may enhance physical improvements in body structure and function, including reduction in spasticity and improvements in gait parameters.
Impact
-
Central nervous system stimulants were examined for efficacy on physical function and spasticity in children with cerebral palsy.
-
The evidence on the effects of central nervous system stimulants on physical function in children with CP is limited and inconsistent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Centers for Disease Control and Prevention. Cerebral Palsy. https://www.cdc.gov/ncbddd/cp/index.html.
Vitrikas, K., Dalton, H. & Breish, D. Cerebral Palsy: An Overview. Am. Fam. Physician 101, 213–220 (2020).
McIntyre, S. et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 64, 1494–1506 (2022).
O’Shea, T. M. Diagnosis, treatment, and prevention of cerebral palsy. Clin. Obstet. Gynecol. 51, 816–828 (2008).
Patel, D. R., Neelakantan, M., Pandher, K. & Merrick, J. Cerebral palsy in children: a clinical overview. Transl. Pediatr. 9, S125–s135 (2020).
Richards, C. L. & Malouin, F. Cerebral palsy: definition, assessment and rehabilitation. Handb. Clin. Neurol. 111, 183–195 (2013).
Musselman, K. E. et al. Prevalence of ataxia in children: a systematic review. Neurology 82, 80–89 (2014).
Delgado, M. R. et al. Efficacy and safety of abobotulinumtoxinA for upper limb spasticity in children with cerebral palsy: a randomized repeat-treatment study. Dev. Med Child Neurol. 63, 592–600 (2021).
Dursun, N. et al. Efficacy of Repeat AbobotulinumtoxinA (Dysport®) Injections in Improving Gait in Children with Spastic Cerebral Palsy. Dev. Neurorehabil 23, 368–374 (2020).
Multani, I., Manji, J., Hastings-Ison, T., Khot, A. & Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Paediatr. Drugs 21, 261–281 (2019).
Strobl, W. et al. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins (Basel) 7, 1629–1648 (2015).
Whelan, M. A. & Delgado, M. R. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology 75, 669 (2010).
Novak, I. et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 20, 3 (2020).
Bohn, E., Goren, K., Switzer, L., Falck-Ytter, Y. & Fehlings, D. Pharmacological and neurosurgical interventions for individuals with cerebral palsy and dystonia: a systematic review update and meta-analysis. Dev. Med Child Neurol. 63, 1038–1050 (2021).
Hägglund, G. et al. Treatment of spasticity in children and adolescents with cerebral palsy in Northern Europe: a CP-North registry study. BMC Neurol. 21, 276–276 (2021).
Goyal, V., Laisram, N., Wadhwa, R. K. & Kothari, S. Y. Prospective Randomized Study of Oral Diazepam and Baclofen on Spasticity in Cerebral Palsy. J. Clin. Diagn. Res 10, RC01–RC05 (2016).
Yadav, S., Chand, S., Majumdar, R. & Sud, A. Effect of botulinum toxin type-A in spasticity and functional outcome of upper limbs in cerebral palsy. J. Clin. Orthop. Trauma 11, 208–212 (2020).
Flemban, A. & Elsayed, W. Effect of combined rehabilitation program with botulinum toxin type A injections on gross motor function scores in children with spastic cerebral palsy. J. Phys. Ther. Sci. 30, 902–905 (2018).
Stray, L. L., Stray, T., Iversen, S., Ruud, A. & Ellertsen, B. Methylphenidate improves motor functions in children diagnosed with Hyperkinetic Disorder. Behav. Brain Funct. 5, 21 (2009).
Jacobi-Polishook, T., Shorer, Z. & Melzer, I. The effect of methylphenidate on postural stability under single and dual task conditions in children with attention deficit hyperactivity disorder - a double blind randomized control trial. J. Neurol. Sci. 280, 15–21 (2009).
Boogerd, W. & Beijnen, J. H. Methylphenidate for cerebral palsy with choreoathetosis. Ann. Intern Med 132, 510 (2000).
Gladstone, D. J. et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke 37, 179–185 (2006).
Grade, C., Redford, B., Chrostowski, J., Toussaint, L. & Blackwell, B. Methylphenidate in early poststroke recovery: a double-blind, placebo-controlled study. Arch. Phys. Med Rehabil. 79, 1047–1050 (1998).
Carucci, S. et al. Long term methylphenidate exposure and growth in children and adolescents with ADHD. A systematic review and meta-analysis. Neurosci. Biobehav Rev. 120, 509–525 (2021).
Goldman, R. D. ADHD stimulants and their effect on height in children. Can. Fam. Physician 56, 145–146 (2010).
Nyberg, F. Structural plasticity of the brain to psychostimulant use. Neuropharmacology 87, 115–124 (2014).
Reid, L. B., Rose, S. E. & Boyd, R. N. Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat. Rev. Neurol. 11, 390–400 (2015).
Matusz, P. J. et al. Somatosensory Plasticity in Pediatric Cerebral Palsy following Constraint-Induced Movement Therapy. Neural Plasticity 2018, 1891978 (2018).
Ostry, D. J. & Gribble, P. L. Sensory Plasticity in Human Motor Learning. Trends Neurosci. 39, 114–123 (2016).
Levac, D., Colquhoun, H. & O’Brien, K. K. Scoping studies: advancing the methodology. Implement Sci. 5, 69 (2010).
Arksey, H. & O’Malley, L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32 (2005).
Tricco, A. C. et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med 169, 467–473 (2018).
Gronseth G. S., et al. Clinical Practice Guideline Process Manual. Am. Acad. Neurol. (2017).
Hurst, D. L. & Lajara-Nanson, W. Use of modafinil in spastic cerebral palsy. J. Child Neurol. 17, 169–172 (2002).
Hurst, D. L., Lajara-Nanson, W. A., Dinakar, P. & Schiffer, R. B. Retrospective review of modafinil use for cerebral palsy. J. Child Neurol. 19, 948–951 (2004).
Hurst, D. L., Lajara-Nanson, W. A. & Lance-Fish, M. E. Walking with modafinil and its use in diplegic cerebral palsy: retrospective review. J. Child Neurol. 21, 294–297 (2006).
Murphy, A. M., Milo-Manson, G., Best, A., Campbell, K. A. & Fehlings, D. Impact of modafinil on spasticity reduction and quality of life in children with CP. Dev. Med Child Neurol. 50, 510–514 (2008).
Sadowska, M., Sarecka-Hujar, B. & Kopyta, I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr. Dis. Treat. 16, 1505–1518 (2020).
Volkow, N. D. et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama 301, 1148–1154 (2009).
Schmidt, A. et al. Comparative Effects of Methylphenidate, Modafinil, and MDMA on Response Inhibition Neural Networks in Healthy Subjects. Int J. Neuropsychopharmacol. 20, 712–720 (2017).
Lange, R., Volkmer, M., Heesen, C. & Liepert, J. Modafinil effects in multiple sclerosis patients with fatigue. J. Neurol. 256, 645–650 (2009).
Liepert, J., Allstadt-Schmitz, J. & Weiller, C. Motor excitability and motor behaviour after modafinil ingestion–a double-blind placebo-controlled cross-over trial. J. Neural Transm. (Vienna) 111, 703–711 (2004).
Childress, A. C., Komolova, M. & Sallee, F. R. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin. Drug Metab. Toxicol. 15, 937–974 (2019).
Lokk, J., Salman Roghani, R. & Delbari, A. Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke–a randomized, double-blind, placebo-controlled trial. Acta Neurol. Scand. 123, 266–273 (2011).
Plenger, P. M. et al. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch. Phys. Med Rehabil. 77, 536–540 (1996).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2023-0121”. The author would like to thank Deanship of Scientific Research at Majmaah University for supporting this work under project number “R-2023-827”.
Author information
Authors and Affiliations
Contributions
A.B.A. had a substantial contribution to the conceptualization and design of the article. She also helped with the acquisition, analysis, and interpretation of data. Finally, she drafted the article. N.Z.A. helped with the acquisition, analysis, and interpretation of data. He also revised the draft critically for important intellectual content. L.V. had a substantial contribution to the conceptualization and design of the article. She also revised the draft critically for important intellectual content. M.M.A. had a substantial contribution to the conceptualization and design of the article. He also helped with the acquisition, analysis, and interpretation of data.
Corresponding author
Ethics declarations
Competing interests
The authors report there are no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almutairi, A.B., Alrashdi, N.Z., Vogtle, L. et al. Effect of psychostimulant medications on physical function in children with cerebral palsy: scoping review. Pediatr Res 95, 1217–1223 (2024). https://doi.org/10.1038/s41390-023-02933-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02933-3