Abstract
Introduction
Acute transverse myelitis (ATM) is a rare neurological complication of Coronavirus disease (COVID-19) vaccines. Various vaccines have been linked to ATM, such as non-replicating viral vectors, ribonucleic acid, and inactivated vaccines. An ATM case is presented here involving the BNT162b2 vaccine leading to asymmetrical incomplete paraplegia and neurogenic bladder.
Case presentation
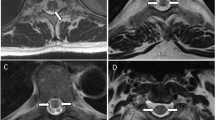
A 66-year-old male developed urinary retention one day after his second dose of the BNT162b2 vaccine, followed by rapidly progressing lower limb weakness. Clinical examination showed asymmetrical paraparesis, reduced sensation below the T8 level, including perianal sensation, and loss of ankle and anal reflexes. Laboratory tests were largely unremarkable, while the spine MRI revealed thickened conus medullaris with a mild increase in T2/STIR signal intensity and subtle enhancement post gadolinium. Following treatment with methylprednisolone, plasmapheresis, and immunoglobulin, and a rehabilitation program, the patient achieved good motor and sensory recovery, but the bladder dysfunction persisted. Single-channel cystometry indicated neurogenic detrusor underactivity and reduced bladder sensation, as evidenced by low-pressure and compliant bladder. The urethral sphincter appeared intact or overactive. The post-void residual urine was significant, necessitating prolonged intermittent catheterisation.
Discussion
Bladder dysfunction due to the COVID-19 vaccine-associated ATM is not as commonly reported as motor or sensory deficits. To our knowledge, this is the first case to highlight a neurogenic bladder that necessitates prolonged intermittent catheterisation as a consequence of COVID-19 vaccine-associated ATM. This report highlights the rare complication of the neurogenic bladder resulting from the BNT162b2 vaccine. Early detection and treatment are crucial to prevent long-term complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Group TMCW. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505.
Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front Immunol. 2021;12:653786.
Maroufi SF, Naderi Behdani F, Rezania F, Tanhapour Khotbehsara S, Mirzaasgari Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: case report and review of literature. Hum Vaccin Immunother. 2022;18:2040239.
Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358:577606.
Tahir N, Koorapati G, Prasad S, Jeelani HM, Sherchan R, Shrestha J, et al. SARS-CoV-2 Vaccination-Induced Transverse Myelitis. Cureus. 2021;13:e16624.
da Gama PD, de Alcantara TG, Smaniotto RR, Petuco PL, et al. Extensive longitudinal transverse myelitis temporally related to the use of AZD1222, AstraZeneca COVID-19 vaccine: cerebrospinal fluid analysis and recent data review. Case Rep Neurol Med. 2022;2022:8999853.
Eom H, Kim SW, Kim M, Kim YE, Kim JH, Shin HY, et al. Case reports of acute transverse myelitis associated With mRNA vaccine for COVID-19. J Korean Med Sci. 2022;37:e52.
Alabkal J, Rebchuk AD, Lyndon D, Randhawa N. Incomplete subacute transverse myelitis following vaccination with Pfizer-BioNTech COVID-19 mRNA vaccine: a case report. Cureus. 2021;13:e20460.
Nakano H, Yamaguchi K, Kawabata K, Asakawa M, Matsumoto Y. Acute transverse myelitis after BNT162b2 vaccination against COVID-19: Report of a fatal case and review of the literature. J Neurol Sci. 2022;434:120102.
Fitzsimmons W, Nance CS Sudden onset of myelitis after COVID-19 vaccination: an under-recognized severe rare adverse event. Available at SSRN 2021 3841558.
Erdem N, Demirci S, Özel T, Mamadova K, Karaali K, Çelik HT, et al. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy Sz. 2021;74:273–6.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
Shoenfeld Y, Aron-Maor A. Vaccination and autoimmunity-‘vaccinosis’: a dangerous liaison? J Autoimmun. 2000;14:1–10.
Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2021;224:108665.
Goriely S, Goldman M. From tolerance to autoimmunity: is there a risk in early life vaccination? J Comp Pathol. 2007;137:S57–61.
Tan WY, Yusof Khan AHK, Mohd Yaakob MN, Abdul Rashid AM, Loh WC, Baharin J, et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol. 2021;21:395.
Notghi AA, Atley J, Silva M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin Med (Lond). 2021;21:e535–e8.
Gajewski JB, Schurch B, Hamid R, Averbeck M, Sakakibara R, Agrò EF, et al. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2018;37:1152–61.
Innovation NAfC. Management of the Neurogenic Bladder for Adults with Spinal Cord Injuries2013 [cited 2023 August 13]. Available from: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0010/155179/ACI-Management-neurogenic-bladder-adults-sci.pdf.
Author information
Authors and Affiliations
Contributions
MFZ was responsible for the formulation of the concept and design of the study, literature search, data collection and processing, analysis, and interpretation of the results, writing the manuscript, preparation of the table and figure, and reviewing and approving the final version of the manuscript. MRH was responsible for the design of the study, data collection and processing, analysis, and interpretation of the results, writing the manuscript, preparation of the table and figure, and reviewing and approving the final version of the manuscript. CEM was responsible for the design of the study, data collection and processing, analysis, and interpretation of the results, writing the manuscript, preparation of the table and figure, and reviewing and approving the final version of the manuscript. TC was responsible for the formulation of the concept and design of the study, literature search, data collection and processing, and interpretation of the results, writing the manuscript, and reviewing and approving the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research has been conducted with the ethical approval of the National Medical Research Register Malaysia (NMRR ID-23-00250-GHX). Informed consent was also obtained from the patient for the publication of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zainudin, M.F., Hasim, M.R., Martin, C.E. et al. A report on neurogenic bladder in COVID-19 vaccine-associated acute transverse myelitis. Spinal Cord Ser Cases 10, 30 (2024). https://doi.org/10.1038/s41394-024-00642-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-024-00642-5