Abstract
Background/objectives
Hypoxia-inducible factor (HIF)-3α1’s role in colorectal cancer (CRC) cells, especially its effects on epithelial-mesenchymal transition (EMT), zinc finger E-box binding homeobox 2 (ZEB2) gene expression, and iron metabolism, remains largely unstudied. This research sought to elucidate these relationships.
Methods
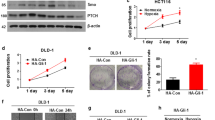
RNA-seq was conducted to investigate the impact of HIF-3α1 overexpression in CRC cells. Dual-luciferase reporter assays assessed the direct targeting of ZEB2 by HIF-3α1. Scratch assays measured changes in cell migration following HIF-3α1 overexpression and ZEB2 knockdown. The effects of HIF-3α1 overexpression on colon tumour growth and liver metastasis were examined in vivo. Iron chelation was used to explore the role of iron metabolism in HIF-3α1-mediated EMT and tumour growth.
Results
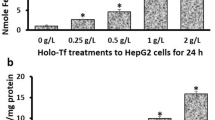
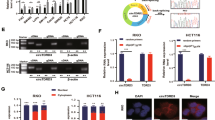
HIF-3α1 overexpression induced EMT and upregulated ZEB2 expression, enhancing cancer cell migration. ZEB2 knockdown reduced mesenchymal markers and cell migration. HIF-3α1 promoted colon tumour growth and liver metastasis, increased transferrin receptor (TFRC) expression and cellular iron levels, and downregulated HIF-1α, HIF-2α, and NDRG1. Iron chelation mitigated HIF-3α1-mediated EMT, tumour growth, and survival.
Conclusions
HIF-3α1 plays a critical role in colon cancer progression by promoting EMT, iron accumulation, and metastasis through ZEB2 and TFRC regulation, suggesting potential therapeutic targets in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
For original data, please contact xxue@salud.unm.edu.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, et al. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol 2017;3:163–73.
Manfredi S, Lepage C, Hatem C, Coatmeyr O, Faivre J, Bouvier A. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254–9.
Leith JT, Padfield G, Faulkner L, Michelson S. Hypoxic fractions in xenografted human colon tumors. Cancer Res. 1991;51:5139–43.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408.
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol 2011;8:393.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig 2009;119:1420–8.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Investig 2009;119:1429–37.
Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–8.
Ravenna L, Salvatori L, Russo MA. HIF3α: the little we know. FEBS J. 2016;283:993–1003.
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5.
Imamura T, Kikuchi H, Herraiz M, Park D, Mizukami Y, Mino-Kenduson M, et al. HIF-1alpha and HIF-2alpha have divergent roles in colon cancer. Int J Cancer. 2009;124:763–71.
Nagaraju GP, Bramhachari PV, Raghu G, El-Rayes BF. Hypoxia inducible factor-1α: Its role in colorectal carcinogenesis and metastasis. Cancer Lett. 2015;366:11–18.
Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye J, et al. Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis. PLoS ONE. 2013;8:e80337.
Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153:505–20.
Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, et al. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72:2285–93.
Xue X, Ramakrishnan SK, Shah YM. Activation of HIF-1α does not increase intestinal tumorigenesis. Am J Physiol Gastrointest Liver Physiol 2014;307:G187–95.
Xue X, Jungles K, Onder G, Samhoun J, Győrffy B, Hardiman K. HIF-3α1 promotes colorectal tumor cell growth by activation of JAK-STAT3 signaling. Oncotarget. 2016;7:11567–79.
Morales M, Xue X. Targeting iron metabolism in cancer therapy. Theranostics. 2021;11:8412–29.
Kim H, Villareal LB, Liu Z, Haneef M, Falcon DM, Martin DR, et al. Transferrin receptor-mediated iron uptake promotes colon tumorigenesis. Adv Sci. 2023;10:2207693.
Schwartz AJ, Goyert JW, Solanki S, Kerk SA, Chen B, Castillo C, et al. Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat Metab 2021;3:969–82.
Mehta KJ, Sharp PA. Iron elevates mesenchymal and metastatic biomarkers in HepG2 cells. Sci Rep. 2020;10:21926.
Xue X, Ramakrishnan S, Weisz K, Triner D, Xie L, Győrffy B, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016;24:447–61.
Triner D, Xue X, Schwartz AJ, Jung I, Colacino JA, Shah YM. Epithelial hypoxia-inducible factor 2α facilitates the progression of colon tumors through recruiting neutrophils. Mol Cell Biol. 2017;37:e00481–16.
Yin K, Lee J, Liu Z, Kim H, Martin DR, Wu D, et al. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell Death Differ. 2021;28:2421–35.
Beltran M, Puig I, Peña C, García JM, Alvarez AB, Peña R, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev 2008;22:756–69.
Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–41.
Jędroszka D, Orzechowska M, Hamouz R, Górniak K, Bednarek AK. Markers of epithelial-to-mesenchymal transition reflect tumor biology according to patient age and Gleason score in prostate cancer. PLoS ONE. 2017;12:e0188842.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to mesenchymal transition. J Clin Investig 2007;117:3810–20.
Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, et al. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS ONE. 2015;10:e0129603.
Bartha Á, Győrffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22:2622.
Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, et al. A preclinical murine model of hepatic metastases. J Vis Exp. 2014; https://doi.org/10.3791/51677.
Wang Y, Yu L, Ding J, Chen Y. Iron metabolism in cancer. Int J Mol Sci. 2018;20:95.
Bhutia YD, Ogura J, Grippo PJ, Torres C, Sato T, Wachtel M, et al. Chronic exposure to excess iron promotes EMT and cancer via p53 loss in pancreatic cancer. Asian J Pharm Sci. 2020;15:237–51.
Bianchi L, Tacchini L, Cairo G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999;27:4223–7.
Fang H-Y, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–59.
Fong G-H, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008;15:635–41.
Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–6.
Wang Q, Li L-H, Gao G-D, Wang G, Qu L, Li J-G, et al. HIF-1α up-regulated NDRG1 expression through binding to NDRG1 promoter, leading to proliferation of lung cancer A549 cells. Mol Biol Rep. 2013;40:3723–9.
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem. 2012;287:17016–28.
Miess H, Dankworth B, Gouw AM, Rosenfeldt M, Schmitz W, Jiang M, et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene. 2018;37:5435–50.
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617.
Gutierrez E, Richardson DR, Jansson PJ. The anticancer agent Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes prosurvival autophagy by two mechanisms. J Biol Chem. 2014;289:33568–89.
Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 2005;23:8490–9.
Tsikitis VL, Larson DL, Wolff BG, Kennedy G, Diehl N, Qin R, et al. Survival in stage III colon cancer is independent of the total number of lymph nodes retrieved. J Am Coll Surg 2009;208:42–47.
Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–97.
Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12.
Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74.
Zhang P, Yao Q, Lu L, Li Y, Chen P-J, Duan C, et al. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6:1110–21.
Mi L, Zhu F, Yang X, Lu J, Zheng Y, Zhao Q, et al. The metastatic suppressor NDRG1 inhibits EMT, migration and invasion through interaction and promotion of caveolin-1 ubiquitylation in human colorectal cancer cells. Oncogene. 2017;36:4323–35.
Yang S-L, Wu C, Xiong Z-F, Fang X. Progress on hypoxia-inducible factor-3: its structure, gene regulation and biological function (Review). Mol Med Rep. 2015;12:2411–6.
Vivinetto AL, Kim I-D, Goldberg DC, Fones L, Brown E, Tarabykin VS, et al. Zeb2 is a regulator of astrogliosis and functional recovery after CNS injury. Cell Rep. 2020;31:107834.
Cao L-L, Liu H, Yue Z, Liu L, Pei L, Gu J, et al. Iron chelation inhibits cancer cell growth and modulates global histone methylation status in colorectal cancer. Biometals. 2018;31:797–805.
Wang W, Tabu K, Aimaitijiang A, Taga T. Therapy-resistant nature of cancer stem cells in view of iron metabolism. Inflamm Regen. 2022;42:34.
Funding
XX was supported by the National Institutes of Health (P20 GM130422, K01DK114390), a Research Scholar Grant from the American Cancer Society (RSG-18-050-01-NEC), Environmental Health and Toxicology Pilot Awards from UNM Center for Native Environmental Health Equity Research (P50 MD015706) and New Mexico Integrative Science Program Incorporating Research in Environmental Sciences (1P30ES032755), Shared Resources and Research Program Support Pilot Project Awards from UNM comprehensive cancer center (P30CA118100), a Cardiovascular and Metabolic Disease Research Program Pilot Project Grant from UNMHSC Office of Research Signature Programs and the Dedicated Health Research Funds from Research Allocation Committee at the University of New Mexico School of Medicine. LV was partially supported by a Predoctoral Fellowship from the NIAID-funded Biology of Infectious Disease and Inflammation program (T32AI007538). DMF was supported by Academic Science Education and Research Training (ASERT) from NIGMS (K12-GM088021).
Author information
Authors and Affiliations
Contributions
XX designed the research, LBV and DMF performed the research, LX contributed vital analytical tools, and LBV and XX wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal studies were carried out in accordance with the Institute of Laboratory Animal Resources guidelines and approved by the University Committee on the Use and Care of Animals at the University of New Mexico (Protocol# 20-201060-HSC).
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Villareal, L.B., Falcon, D.M., Xie, L. et al. Hypoxia-inducible factor 3α1 increases epithelial-to-mesenchymal transition and iron uptake to drive colorectal cancer liver metastasis. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02699-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02699-3