Abstract
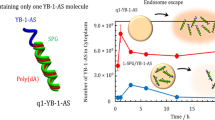
Antisense oligonucleotides (AS-ODNs) hybridize with specific mRNAs, resulting in interference with the splicing mechanism or the regulation of protein translation. We previously demonstrated that the β-glucan schizophyllan (SPG) can form a complex with AS-ODNs with attached dA40 (AS-ODNs/SPG), and this complex can be incorporated into cells, such as macrophages and dendritic cells, expressing the β-glucan receptor Dectin-1. We have achieved efficient gene silencing in animal models, but the uptake mechanism and intracellular distribution are unclear. In this study, we prepared the complex consisting of SPG and AS-ODNs (AS014) for Y-box binding protein-1 (YB-1). After treatment with endocytosis inhibitor Pitstop 2 and small interfering RNA targeting Dectin-1, we found that AS014/SPG complexes are incorporated into cells by Dectin-1-mediated endocytosis and inhibit cell growth in a Dectin-1 expression level-dependent manner. After treatment with AS014/SPG complexes, we separated the cell lysate into endosomal and cytoplasmic components by ultracentrifugation and directly determined the distribution of AS014 by reverse transcription PCR using AS014 ODNs as a template or a reverse transcription primer. In the cytoplasm, AS014 clearly hybridized with YB-1 mRNAs. This is the first demonstration of the distinct distribution of the complex in cells. These results could facilitate the clinical application of the complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hagedorn PH, Hansen BR, Koch T, Lindow M. Managing the sequence-specificity of antisense oligonucleotides in drug discovery. Nucleic Acids Res. 2017;45:2262–82.
Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA. 1978;75:280–4.
Dean NM, Bennett CF. Antisense oligonucleotide-based therapeutics for cancer. Oncogene. 2003;22:9087–96.
Coppelli FM, Grandis JR. Oligonucleotides as anticancer agents: from the benchside to the clinic and beyond. Curr Pharm Des. 2005;11:2825–40.
Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol. 2006;33:533–40.
Roehr B. Fomivirsen approved for CMV retinitis. J Int Assoc Physicians AIDS Care. 1998;4:14–6.
Kapteyn J, Hoyer L, Hecht J, Müller W, Andel A, Verkleij A, et al. The cell wall architecture of Candida albicans wild‐type cells and cell wall‐defective mutants. Mol Microbiol. 2000;35:601–11.
Kim Y-T, Kim E-H, Cheong C, Williams DL, Kim C-W, Lim S-T. Structural characterization of β-D-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydr Res. 2000;328:331–41.
Klis F, Groot PD, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39:1–8.
Sakurai K, Mizu M, Shinkai S. Polysaccharide− polynucleotide complexes. 2. Complementary polynucleotide mimic behavior of the natural polysaccharide schizophyllan in the macromolecular complex with single-stranded RNA and DNA. Biomacromolecules. 2001;2:641–50.
Miyoshi K, Uezu K, Sakurai K, Shinkai S. Proposal of a new hydrogen‐bonding form to maintain curdlan triple helix. Chem Biodivers. 2004;1:916–24.
Numata M, Asai M, Kaneko K, Bae A-H, Hasegawa T, Sakurai K, et al. Inclusion of cut and as-grown single-walled carbon nanotubes in the helical superstructure of schizophyllan and curdlan (β-1, 3-glucans). J Am Chem Soc. 2005;127:5875–84.
Brown GD, Gordon S. Immune recognition: a new receptor for β-glucans. Nature. 2001;413:36–37.
Taylor PR, Brown GD, Geldhof AB, Martinez‐Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–7.
Mochizuki S, Sakurai K. Dectin-1 targeting delivery of TNF-α antisense ODNs complexed with β-1, 3-glucan protects mice from LPS-induced hepatitis. J Control Release. 2011;151:155–61.
Izumi H, Nagao S, Mochizuki S, Fujiwara N, Sakurai K, Morimoto Y. Optimal sequence of antisense DNA to silence YB-1 in lung cancer by use of a novel polysaccharide drug delivery system. Int J Oncol. 2016;48:2472–8.
Mochizuki S, Morishita H, Sakurai K. Macrophage specific delivery of TNF-α siRNA complexed with β-1, 3-glucan inhibits LPS-induced cytokine production in a murine acute hepatitis model. Bioorg Med Chem. 2013;21:2535–42.
Zhang Q, Ichimaru N, Higuchi S, Cai S, Hou J, Fujino M, et al. Permanent acceptance of mouse cardiac allografts with CD40 siRNA to induce regulatory myeloid cells by use of a novel polysaccharide siRNA delivery system. Gene Ther. 2015;22:217–26.
Takedatsu H, Mitsuyama K, Mochizuki S, Kobayashi T, Sakurai K, Takeda H, et al. A new therapeutic approach using a schizophyllan-based drug delivery system for inflammatory bowel disease. Mol Ther. 2012;20:1234–41.
Heyl KA, Klassert TE, Heinrich A, Müller MM, Klaile E, Dienemann H, et al. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. mBio. 2014;5:e01492–14.
Uchiumi T, Fotovati A, Sasaguri T, Shibahara K, Shimada T, Fukuda T, et al. YB-1 Is important for an early stage embryonic development neural tube formation and cell proliferation. J Biol Chem. 2006;281:40440–9.
Bargou RC, Jürchott K, Wagener C, Bergmann S, Metzner S, Bommert K, et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat Med. 1997;3:447–50.
Oda Y, Sakamoto A, Shinohara N, Ohga T, Uchiumi T, Kohno K, et al. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human osteosarcoma. Clin Cancer Res. 1998;4:2273–7.
Shibao K, Takano H, Nakayama Y, Okazaki K, Nagata N, Izumi H, et al. Enhanced coexpression of YB‐1 and DNA topoisomerase II α genes in human colorectal carcinomas. Int J Cancer. 1999;83:732–7.
Basaki Y, Taguchi K-i, Izumi H, Murakami Y, Kubo T, Hosoi F, et al. Y-box binding protein-1 (YB-1) promotes cell cycle progression through CDC6-dependent pathway in human cancer cells. Eur J Cancer. 2010;46:954–65.
Shibahara K, Sugio K, Osaki T, Uchiumi T, Maehara Y, Kohno K, et al. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin Cancer Res. 2001;7:3151–5.
Kuwano M, Uchiumi T, Hayakawa H, Ono M, Wada M, Izumi H, et al. The basic and clinical implications of ABC transporters, Y‐box‐binding protein‐1 (YB‐1) and angiogenesis‐related factors in human malignancies. Cancer Sci. 2003;94:9–14.
Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, et al. Twist promotes tumor cell growth through YB-1 expression. Cancer Res. 2008;68:98–105.
von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–84.
McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–33.
Sanada Y, Matsuzaki T, Mochizuki S, Okobira T, Uezu K, Sakurai K. β-1,3-D-glucan schizophyllan/poly (dA) triple-helical complex in dilute solution. J Phys Chem B. 2011;116:87–94.
Su X, Fricke J, Kavanagh DG, Irvine DJ. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8:774–87.
Sershen S, Westcott S, Halas N, West J. Temperature‐sensitive polymer–nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res A. 2000;51:293–8.
Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935–9.
Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, et al. Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano. 2012;6:9335–46.
Mochizuki S, Morishita H, Adachi Y, Yamaguchi Y, Sakurai K. Binding assay between murine Dectin-1 and beta-glucan/DNA complex with quartz-crystal microbalance. Carbohydr Res. 2014;391:1–8.
Adachi Y, Ishii T, Ikeda Y, Hoshino A, Tamura H, Aketagawa J, et al. Characterization of β-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72:4159–71.
Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–54.
Raja RH, McGary CT, Weigel PH. Affinity and distribution of surface and intracellular hyaluronic acid receptors in isolated rat liver endothelial cells. J Biol Chem. 1988;263:16661–8.
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–301.
Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, Patri VS, Kim JM, et al. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjug Chem. 2009;20:47–59.
Minari J, Mochizuki S, Matsuzaki T, Adachi Y, Ohno N, Sakurai K. Enhanced cytokine secretion from primary macrophages due to Dectin-1 mediated uptake of CpG DNA/β-1, 3-glucan complex. Bioconjug Chem. 2010;22:9–15.
Mochizuki S, Morishita H, Sakurai K. Complex consisting of β-glucan and antigenic peptides with cleavage site for glutathione and aminopeptidases induces potent cytotoxic T lymphocytes. Bioconjug Chem. 2017;28:2246–53.
Mochizuki S, Morishita H, Kobiyama K, Aoshi T, Ishii KJ, Sakurai K, Immunization with antigenic peptides complexed with beta-glucan induces potent cytotoxic T-lymphocyte activity in combination with CpG-ODNs. J Control Release. 2015;220 (Pt A):495–502.
Acknowledgements
This work was supported by the JST CREST Grant Number JPMJCR1521, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fujiwara, N., Izumi, H., Morimoto, Y. et al. Complex consisting of antisense DNA and β-glucan promotes internalization into cell through Dectin-1 and hybridizes with target mRNA in cytosol. Cancer Gene Ther 26, 32–40 (2019). https://doi.org/10.1038/s41417-018-0033-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0033-2
This article is cited by
-
Antisense DNA cocktail therapy using short ß-1,3-glucan/oligonucleotide complexes
Polymer Journal (2023)
-
Delivery of therapeutic oligonucleotides targeting Dectin-1 using quantized complexes
Polymer Journal (2022)
-
RNA-based therapies: A cog in the wheel of lung cancer defense
Molecular Cancer (2021)