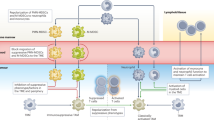
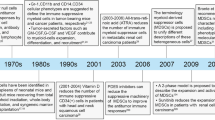
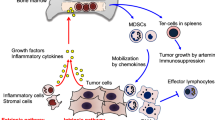
Abstract
Anticancer agents continue to dominate the list of newly approved drugs, approximately half of which are immunotherapies. This trend illustrates the considerable promise of cancer treatments that modulate the immune system. However, the immune system is complex and dynamic, and can have both tumour-suppressive and tumour-promoting effects. Understanding the full range of immune modulation in cancer is crucial to identifying more effective treatment strategies. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid cells that develop in association with chronic inflammation, which is a hallmark of cancer. Indeed, MDSCs accumulate in the tumour microenvironment, where they strongly inhibit anticancer functions of T cells and natural killer cells and exert a variety of other tumour-promoting effects. Emerging evidence indicates that MDSCs also contribute to resistance to cancer treatments, particularly immunotherapies. Conversely, treatment approaches designed to eliminate cancer cells can have important additional effects on MDSC function, which can be either positive or negative. In this Review, we discuss the interplay between MDSCs and various other cell types found in tumours as well as the mechanisms by which MDSCs promote tumour progression. We also discuss the relevance and implications of MDSCs for cancer therapy.
Key points
-
Cancer-associated conditions, including hypoxia, nutrient deficiency, acidity, endoplasmic reticulum stress and long-term production of inflammatory mediators, promote the generation of myeloid-derived suppressor cells (MDSCs).
-
MDSCs support the progression of most cancer entities by suppressing antitumour immune responses, stimulating angiogenesis and fostering metastasis through a variety of mechanisms.
-
MDSCs contribute to primary and acquired resistance to cancer immunotherapy; thus, combinatorial therapeutic targeting of these cells might improve patient outcomes.
-
Other cancer treatments, such as chemotherapy, radiotherapy, targeted therapies or hormone therapies, can have distinct and disparate effects on MDSCs and should be studied further.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Condamine, T., Mastio, J. & Gabrilovich, D. I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 98, 913–922 (2015).
Hicks, K. C., Tyurina, Y. Y., Kagan, V. E. & Gabrilovich, D. I. Myeloid cell-derived oxidized lipids and regulation of the tumor microenvironment. Cancer Res. 82, 187–194 (2022).
Gabrilovich, D. I. et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 67, 425 (2007).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Mishalian, I., Granot, Z. & Fridlender, Z. G. The diversity of circulating neutrophils in cancer. Immunobiology 222, 82–88 (2017).
Hegde, S., Leader, A. M. & Merad, M. MDSC: markers, development, states, and unaddressed complexity. Immunity 54, 875–884 (2021).
Antuamwine, B. B. et al. N1 versus N2 and PMN-MDSC: a critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol. Rev. 314, 250–279 (2023).
Ostrand-Rosenberg, S., Lamb, T. J. & Pawelec, G. Here, there, and everywhere: myeloid-derived suppressor cells in immunology. J. Immunol. 210, 1183–1197 (2023).
Groth, C. et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 120, 16–25 (2019).
Özkan, B., Lim, H. & Park, S.-G. Immunomodulatory function of myeloid-derived suppressor cells during B cell-mediated immune responses. Int. J. Mol. Sci. 19, 1468 (2018).
Ma, T. et al. Myeloid-derived suppressor cells in solid tumors. Cells 11, 310 (2022).
Condamine, T., Ramachandran, I., Youn, J.-I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015).
Safarzadeh, E., Orangi, M., Mohammadi, H., Babaie, F. & Baradaran, B. Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis. J. Cell. Physiol. 233, 3024–3036 (2018).
Li, T. et al. Targeting MDSC for immune-checkpoint blockade in cancer immunotherapy: current progress and new prospects. Clin. Med. Insights Oncol. 15, 11795549211035540 (2021).
Bronte, V. et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 96, 3838–3846 (2000).
Bronte, V. et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 161, 5313–5320 (1998).
Movahedi, K. et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111, 4233–4244 (2008).
Haile, L. A., Gamrekelashvili, J., Manns, M. P., Korangy, F. & Greten, T. F. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 185, 203–210 (2010).
Dumitru, C. A., Moses, K., Trellakis, S., Lang, S. & Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 61, 1155–1167 (2012).
Mastio, J. et al. Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs. J. Exp. Med. 216, 2150–2169 (2019).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Alshetaiwi, H. et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 5, eaay6017 (2020).
Condamine, T. et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 1, aaf8943 (2016).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Katzenelenbogen, Y. et al. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 182, 872–885.e19 (2020).
Condamine, T. & Gabrilovich, D. I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 (2011).
Welte, T. et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 18, 632–644 (2016).
Gabrilovich, D. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92, 4150–4166 (1998).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Rivera, L. B. & Bergers, G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 36, 240–249 (2015).
Weber, R. et al. IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma. J. Immunother. Cancer 8, e000949 (2020).
Weber, R. et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell. Immunol. 359, 104254 (2021).
Corzo, C. A. et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453 (2010).
Obermajer, N., Muthuswamy, R., Lesnock, J., Edwards, R. P. & Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118, 5498–5505 (2011).
Morello, S., Pinto, A., Blandizzi, C. & Antonioli, L. Myeloid cells in the tumor microenvironment: role of adenosine. Oncoimmunology 5, e1108515 (2016).
Fultang, N., Li, X., Li, T. & Chen, Y. H. Myeloid-derived suppressor cell differentiation in cancer: transcriptional regulators and enhanceosome-mediated mechanisms. Front. Immunol. 11, 619253 (2021).
McClure, C. et al. Stat3 and C/EBPβ synergize to induce miR-21 and miR-181b expression during sepsis. Immunol. Cell Biol. 95, 42–55 (2017).
Halaby, M. J. et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci. Immunol. 4, eaax8189 (2019).
Yan, D. et al. TIPE2 specifies the functional polarization of myeloid-derived suppressor cells during tumorigenesis. J. Exp. Med. 217, e20182005 (2019).
Waight, J. D. et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J. Clin. Invest. 123, 4464–4478 (2013).
Li, T. et al. c-Rel is a myeloid checkpoint for cancer immunotherapy. Nat. Cancer 1, 507–517 (2020).
Lim, H. X., Kim, T. S. & Poh, C. L. Understanding the differentiation, expansion, recruitment and suppressive activities of myeloid-derived suppressor cells in cancers. Int. J. Mol. Sci. 21, 3599 (2020).
Groth, C. et al. Blocking migration of polymorphonuclear myeloid-derived suppressor cells inhibits mouse melanoma progression. Cancers 13, 726 (2021).
Blattner, C. et al. CCR5+ myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 78, 157–167 (2018).
Huang, K. et al. Notch3 signaling promotes colorectal tumor growth by enhancing immunosuppressive cells infiltration in the microenvironment. BMC Cancer 23, 55 (2023).
Bitsch, R. et al. STAT3 inhibitor napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J. Immunother. Cancer 10, e004384 (2022).
Arkhypov, I. et al. Myeloid cell modulation by tumor-derived extracellular vesicles. Int. J. Mol. Sci. 21, 6319 (2020).
Xiang, X. et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 124, 2621–2633 (2009).
Condamine, T. et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest. 124, 2626–2639 (2014).
Veglia, F. et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 569, 73–78 (2019).
Pan, P.-Y. et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70, 99–108 (2010).
Zoso, A. et al. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 44, 3307–3319 (2014).
Pang, B. et al. Myeloid-derived suppressor cells shift Th17/Treg ratio and promote systemic lupus erythematosus progression through arginase-1/miR-322-5p/TGF-β pathway. Clin. Sci. 134, 2209–2222 (2020).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Lee-Chang, C. et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol. Res. 7, 1928–1943 (2019).
Bodogai, M. et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 75, 3456–3465 (2015).
Ostrand-Rosenberg, S., Sinha, P., Beury, D. W. & Clements, V. K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 22, 275–281 (2012).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Beury, D. W. et al. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J. Leukoc. Biol. 96, 1109–1118 (2014).
van Vlerken-Ysla, L., Tyurina, Y. Y., Kagan, V. E. & Gabrilovich, D. I. Functional states of myeloid cells in cancer. Cancer Cell 41, 490–504 (2023).
Kwak, T. et al. Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep. 33, 108571 (2020).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Beatty, P. L. et al. Immunobiology and immunosurveillance in patients with intraductal papillary mucinous neoplasms (IPMNs), premalignant precursors of pancreatic adenocarcinomas. Cancer Immunol. Immunother. 65, 771–778 (2016).
Huang, X. et al. The molecular, immune features, and risk score construction of intraductal papillary mucinous neoplasm patients. Front. Mol. Biosci. 9, 887887 (2022).
Ma, P. et al. Circulating myeloid derived suppressor cells (MDSC) that accumulate in premalignancy share phenotypic and functional characteristics with MDSC in cancer. Front. Immunol. 10, 1401 (2019).
Ding, L. et al. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J. Clin. Invest. 126, 2867–2880 (2016).
Ding, L. et al. Toll-like receptor 9 pathway mediates schlafen+ MDSC polarization during helicobacter-induced gastric metaplasias. Gastroenterology 163, 411–425.e4 (2022).
Zhang, X. et al. CD147 mediates epidermal malignant transformation through the RSK2/AP-1 pathway. J. Exp. Clin. Cancer Res. 41, 246 (2022).
Wang, T. et al. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity 49, 504–514.e4 (2018).
Zhang, Q. et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 11, 1248–1267 (2021).
Harusato, A. et al. Early-life microbiota exposure restricts myeloid-derived suppressor cell-driven colonic tumorigenesis. Cancer Immunol. Res. 7, 544–551 (2019).
Campregher, C., Luciani, M. G. & Gasche, C. Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut 57, 780 (2008).
Ibrahim, M. L. et al. Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell Rep. 25, 3036–3046.e6 (2018).
Grivennikov, S. et al. IL-6 and STAT3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Ke, Z. et al. PAR2 deficiency enhances myeloid cell-mediated immunosuppression and promotes colitis-associated tumorigenesis. Cancer Lett. 469, 437–446 (2020).
Tang, C. et al. Blocking Dectin-1 prevents colorectal tumorigenesis by suppressing prostaglandin E2 production in myeloid-derived suppressor cells and enhancing IL-22 binding protein expression. Nat. Commun. 14, 1493 (2023).
Chen, X. et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J. Clin. Invest. 123, 4595–4611 (2013).
Seliger, B. Strategies of tumor immune evasion. BioDrugs 19, 347–354 (2005).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198 (2015).
Hsu, J. et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018).
Sun, R. et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl. Oncol. 13, 100825 (2020).
Christiansson, L. et al. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE 8, e55818 (2013).
Zhang, B. et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 8, e57114 (2013).
Duraiswamy, J., Freeman, G. J. & Coukos, G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 73, 6900–6912 (2013).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Iwata, T. et al. PD-L1+MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci. Rep. 6, 39296 (2016).
Prima, V., Kaliberova, L. N., Kaliberov, S., Curiel, D. T. & Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl Acad. Sci. USA 114, 1117–1122 (2017).
Fleming, V. et al. Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 79, 4715–4728 (2019).
Filipazzi, P., Bürdek, M., Villa, A., Rivoltini, L. & Huber, V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin. Cancer Biol. 22, 342–349 (2012).
Valenti, R. et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-β-mediated suppressive activity on T lymphocytes. Cancer Res. 66, 9290–9298 (2006).
Valenti, R. et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 67, 2912–2915 (2007).
Strauss, L. et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 5, eaay1863 (2020).
Rodriguez, P. C., Quiceno, D. G. & Ochoa, A. C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2006).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Grzywa, T. M. et al. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 11, 938 (2020).
Youn, J.-I., Collazo, M., Shalova, I. N., Biswas, S. K. & Gabrilovich, D. I. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 91, 167–181 (2011).
Baumann, T. et al. Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nat. Immunol. 21, 555–566 (2020).
Srivastava, M. K., Sinha, P., Clements, V. K., Rodriguez, P. & Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70, 68–77 (2010).
Yu, J. et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 190, 3783–3797 (2013).
Bilir, C. & Sarisozen, C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J. Oncol. Sci. 3, 52–56 (2017).
Frumento, G. et al. Tryptophan-derived catabolites are responsible for inhibition of t and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196, 459–468 (2002).
Munn, D. H. et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 (2005).
Mezrich, J. D. et al. An Interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010).
Chiesa, M. D. et al. The tryptophan catabolite l-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125 (2006).
Corzo, C. A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009).
Fiaschi, T. & Chiarugi, P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int. J. Cell Biol. 2012, 762825 (2012).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 (2007).
Feng, S. et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl Acad. Sci. USA 115, 10094–10099 (2018).
Bingisser, R. M., Tilbrook, P. A., Holt, P. G. & Kees, U. R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 160, 5729–5734 (1998).
Wang, Z. et al. A myeloid cell population induced by Freund adjuvant suppresses T-cell-mediated antitumor immunity. J. Immunother. 33, 167–177 (2010).
Gehad, A. E. et al. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J. Invest. Dermatol. 132, 2642–2651 (2012).
Molon, B. et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962 (2011).
Stiff, A. et al. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin. Cancer Res. 24, 1891–1904 (2018).
Tcyganov, E. N. et al. Peroxynitrite in the tumor microenvironment changes the profile of antigens allowing escape from cancer immunotherapy. Cancer Cell 40, 1173–1189.e6 (2022).
Li, J. et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 6, e1320011 (2017).
Hanson, E. M., Clements, V. K., Sinha, P., Ilkovitch, D. & Ostrand-Rosenberg, S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol. 183, 937–944 (2009).
Cozzolino, M. et al. The voltage-gated Hv1 H+ channel is expressed in tumor-infiltrating myeloid-derived suppressor cells. Int. J. Mol. Sci. 24, 6216 (2023).
Huber, V. et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 43, 74–89 (2017).
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Loercher, A. E., Nash, M. A., Kavanagh, J. J., Platsoucas, C. D. & Freedman, R. S. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J. Immunol. 163, 6251–6260 (1999).
Vuk-Pavlović, S. et al. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate 70, 443–455 (2010).
Hart, K. M., Byrne, K. T., Molloy, M. J., Usherwood, E. M. & Berwin, B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front. Immunol. 2, 29 (2011).
Sato, Y. et al. Characterization of the myeloid-derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology 4, e995541 (2015).
Hu, C.-E., Gan, J., Zhang, R.-D., Cheng, Y.-R. & Huang, G.-J. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand. J. Gastroenterol. 46, 156–164 (2011).
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M. & Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response1. J. Immunol. 179, 977–983 (2007).
Steinbrink, K., Graulich, E., Kubsch, S., Knop, J. & Enk, A. H. CD4+ and CD8+ anergic T cells induced by interleukin-10–treated human dendritic cells display antigen-specific suppressor activity. Blood 99, 2468–2476 (2002).
Steinbrink, K., Wölfl, M., Jonuleit, H., Knop, J. & Enk, A. H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159, 4772–4780 (1997).
Serafini, P., Borrello, I. & Bronte, V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16, 53–65 (2006).
Jianyi, D. et al. Myeloid-derived suppressor cells cross-talk with B10 cells by BAFF/BAFF-R pathway to promote immunosuppression in cervical cancer. Cancer Immunol. Immunother. 72, 73–85 (2023).
Filipazzi, P. et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 25, 2546–2553 (2007).
Thomas, D. A. & Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Kobie, J. J. et al. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines1. Cancer Res. 63, 1860–1864 (2003).
Li, H., Han, Y., Guo, Q., Zhang, M. & Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β11. J. Immunol. 182, 240–249 (2009).
Mao, Y. et al. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 20, 4096–4106 (2014).
Burke, M., Choksawangkarn, W., Edwards, N., Ostrand-Rosenberg, S. & Fenselau, C. Exosomes from myeloid-derived suppressor cells carry biologically active proteins. J. Proteome Res. 13, 836–843 (2014).
Tumino, N. et al. Polymorphonuclear myeloid-derived suppressor cells are abundant in peripheral blood of cancer patients and suppress natural killer cell anti-tumor activity. Front. Immunol. 12, 803014 (2022).
Gabrilovich, D. I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 5, 3–8 (2017).
Yang, L. et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 (2004).
Du, R. et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13, 206–220 (2008).
Ahn, G. O. & Brown, J. M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13, 193–205 (2008).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Qu, X., Zhuang, G., Yu, L., Meng, G. & Ferrara, N. Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J. Biol. Chem. 287, 19574–19584 (2012).
Kujawski, M. et al. Stat3 mediates myeloid cell–dependent tumor angiogenesis in mice. J. Clin. Invest. 118, 3367–3377 (2008).
Bauer, R. et al. Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of antiangiogenic therapy. Cancer Res. 78, 3220–3232 (2018).
Boelte, K. C. et al. Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PLoS ONE 6, e18534 (2011).
Li, X. et al. Myeloid-derived suppressor cells promote epithelial ovarian cancer cell stemness by inducing the CSF2/p-STAT3 signalling pathway. FEBS J. 287, 5218–5235 (2020).
Cui, T. X. et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 39, 611–621 (2013).
Luo, A. et al. Myeloid-derived suppressor cells recruited by chemokine (C-C motif) ligand 3 promote the progression of breast cancer via phosphoinositide 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J. Breast Cancer 23, 141–161 (2020).
Ai, L. et al. Myeloid-derived suppressor cells endow stem-like qualities to multiple myeloma cells by inducing piRNA-823 expression and DNMT3B activation. Mol. Cancer 18, 88 (2019).
Sangaletti, S. et al. SPARC is a new myeloid-derived suppressor cell marker licensing suppressive activities. Front. Immunol. 10, 1369 (2019).
Panni, R. Z. et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol. Immunother. 63, 513–528 (2014).
Peng, D. et al. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 76, 3156–3165 (2016).
Oh, K. et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 15, R79 (2013).
Toh, B. et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 9, e1001162 (2011).
Ouzounova, M. et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 8, 14979 (2017).
Lin, Y. et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology 75, 28–42 (2022).
Kuroda, H. et al. Prostaglandin E2 produced by myeloid-derived suppressive cells induces cancer stem cells in uterine cervical cancer. Oncotarget 9, 36317–36330 (2018).
Komura, N. et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol. Immunother. 69, 2477–2499 (2020).
Douglass, S. M. et al. Myeloid-derived suppressor cells are a major source of Wnt5A in the melanoma microenvironment and depend on Wnt5A for full suppressive activity. Cancer Res. 81, 658–670 (2021).
Zhang, R. et al. PMN-MDSCs modulated by CCL20 from cancer cells promoted breast cancer cell stemness through CXCL2–CXCR2 pathway. Signal. Transduct. Target. Ther. 8, 97 (2023).
Wang, Y. et al. Granulocytic myeloid-derived suppressor cells promote the stemness of colorectal cancer cells through exosomal S100A9. Adv. Sci. 6, 1901278 (2019).
Yang, L. et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 (2008).
Pickup, M. W. et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res. 73, 5336–5346 (2013).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010).
Hsu, Y.-L. et al. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 21, 23 (2019).
Spiegel, A. et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 6, 630–649 (2016).
Teijeira, Á. et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 52, 856–871.e8 (2020).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Sprouse, M. L. et al. PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/Notch/Nodal signaling. Int. J. Mol. Sci. 20, 1916 (2019).
Cao, Y. et al. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 74, 5091–5102 (2014).
Sceneay, J., Parker, B. S., Smyth, M. J. & Möller, A. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. Oncoimmunology 2, e22355 (2013).
Wang, D., Sun, H., Wei, J., Cen, B. & DuBois, R. N. CXCL1 Is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 77, 3655–3665 (2017).
Wang, Y., Ding, Y., Guo, N. & Wang, S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front. Immunol. 10, 172 (2019).
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Shi, H. et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression. Int. J. Cancer 140, 1370–1383 (2017).
Hiratsuka, S. et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Lim, S. Y. et al. Cd11b+ myeloid cells support hepatic metastasis through down-regulation of angiopoietin-like 7 in cancer cells. Hepatology 62, 521–533 (2015).
Gao, D. et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 72, 1384–1394 (2012).
Catena, R. et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 3, 578–589 (2013).
Shi, H., Li, K., Ni, Y., Liang, X. & Zhao, X. Myeloid-derived suppressor cells: implications in the resistance of malignant tumors to T cell-based immunotherapy. Front. Cell Dev. Biol. 9, 707198 (2021).
Sánchez-León, M. L. et al. The effects of dendritic cell-based vaccines in the tumor microenvironment: impact on myeloid-derived suppressor cells. Front. Immunol. 13, 1050484 (2022).
Li, X. et al. Targeting myeloid-derived suppressor cells to enhance the antitumor efficacy of immune checkpoint blockade therapy. Front. Immunol. 12, 754196 (2021).
Schoenfeld, A. J. & Hellmann, M. D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell 37, 443–455 (2020).
Petrova, V. et al. Immunosuppressive capacity of circulating MDSC predicts response to immune checkpoint inhibitors in melanoma patients. Front. Immunol. 14, 1065767 (2023).
Limagne, E. et al. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology 8, e1564505 (2019).
Theivanthiran, B. et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J. Clin. Invest. 130, 2570–2586 (2020).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Weber, R. et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front. Immunol. 9, 1310 (2018).
Wu, Y., Yi, M., Niu, M., Mei, Q. & Wu, K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol. Cancer 21, 184 (2022).
Zhang, Z. et al. A drug screening to identify novel combinatorial strategies for boosting cancer immunotherapy efficacy. J. Transl. Med. 21, 23 (2023).
Bendell, J. et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 72, 2443–2458 (2023).
Kondo, S. et al. Safety, tolerability, pharmacokinetics, and antitumour activity of oleclumab in Japanese patients with advanced solid malignancies: a phase I, open-label study. Int. J. Clin. Oncol. 27, 1795–1804 (2022).
Zhang, H. et al. Glycoengineered anti-CD39 promotes anticancer responses by depleting suppressive cells and inhibiting angiogenesis in tumor models. J. Clin. Invest. 132, e157431 (2022).
Jahani, V., Yazdani, M., Badiee, A., Jaafari, M. R. & Arabi, L. Liposomal celecoxib combined with dendritic cell therapy enhances antitumor efficacy in melanoma. J. Control. Rel. 354, 453–464 (2023).
Gandhi, S. et al. 547 Safety and efficacy of de-escalated neoadjuvant chemoimmunotherapy of triple negative breast cancer (TNBC) using chemokine-modulating regimen (rintatolimod, IFN-α2b, celecoxib). J. Immunother. Cancer 10, A572 (2022).
Kjeldsen, J. W. et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 27, 2212–2223 (2021).
Long, G. V. et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20, 1083–1097 (2019).
Fleet, J. C., Burcham, G. N., Calvert, R. D., Elzey, B. D. & Ratliff, T. L. 1α, 25 Dihydroxyvitamin D (1,25(OH)2D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC). J. Steroid Biochem. Mol. Biol. 198, 105557 (2020).
Nefedova, Y. et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 67, 11021–11028 (2007).
Li, L. et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 78, 1779–1791 (2018).
Qin, G. et al. Metformin blocks myeloid-derived suppressor cell accumulation through AMPK-DACH1-CXCL1 axis. Oncoimmunology 7, e1442167 (2018).
Dominguez, G. A. et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin. Cancer Res. 23, 2942–2950 (2017).
Tavazoie, M. F. et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e18 (2018).
Cheng, P. et al. Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol. Ther. 30, 2315–2326 (2022).
Fultang, L. et al. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. eBioMedicine 47, 235–246 (2019).
Garg, A. D. et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology 6, e1386829 (2017).
Welters, M. J. et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 8, 334ra352 (2016).
Dijkgraaf, E. M. et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget 6, 32228–32243 (2015).
Vincent, J. et al. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Sevko, A. et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J. Immunol. 190, 2464–2471 (2013).
Gebhardt, C. et al. Potential therapeutic effect of low-dose paclitaxel in melanoma patients resistant to immune checkpoint blockade: a pilot study. Cell. Immunol. 360, 104274 (2021).
Huijts, C. M. et al. The effect of everolimus and low-dose cyclophosphamide on immune cell subsets in patients with metastatic renal cell carcinoma: results from a phase I clinical trial. Cancer Immunol. Immunother. 68, 503–515 (2019).
Hossain, F. et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 3, 1236–1247 (2015).
Perrot, I. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27, 2411–2425.e9 (2019).
Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 (2009).
Deng, Z. et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene 36, 639–651 (2017).
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Zahoor, H. et al. Phase II trial of continuous treatment with sunitinib in patients with high-risk (BCG-refractory) non-muscle invasive bladder cancer. Invest. New Drugs 37, 1231–1238 (2019).
Overman, M. et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J. Immunother. Cancer 8, e000587 (2020).
Refaat, Y., Rahman, Y. A., Mohammed Saleh, M. F., Sayed, D. M. & Elzohri, M. H. Tyrosine kinase inhibitors reduce myeloid-derived suppressor cells in patients with chronic myeloid leukemia with better outcome. Egypt. J. Haematol. 47, 272–280 (2022).
Schilling, B. et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int. J. Cancer 133, 1653–1663 (2013).
Ghonim, M. A. et al. Targeting PARP-1 with metronomic therapy modulates MDSC suppressive function and enhances anti-PD-1 immunotherapy in colon cancer. J. Immunother. Cancer 9, e001643 (2021).
Ibba, S. V., Luu, H. H. & Boulares, A. H. Differential effects of poly(ADP ribose) polymerase inhibitor-based metronomic therapy on programmed death-ligand 1 and matrix-associated factors in human myeloid cells. Am. J. Transl. Res. 14, 9025–9030 (2022).
Tomita, Y. et al. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: correlative analysis of ENCORE 301, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. Oncoimmunology 5, e1219008 (2016).
Lu, Z. et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature 579, 284–290 (2020).
Wang, Q. et al. Reversing myeloid-derived suppressor cells mediated immunosuppression via p38α inhibition enhances immunotherapy efficacy in triple negative breast cancer. Preprint at bioRxiv https://doi.org/10.1101/2023.03.31.535102 (2023).
Cao, M. et al. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab. Invest. 91, 598–608 (2011).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Mao, L. et al. Immunogenic hypofractionated radiotherapy sensitising head and neck squamous cell carcinoma to anti-PD-L1 therapy in MDSC-dependent manner. Br. J. Cancer 128, 11 (2023).
Iliadi, C. et al. The current understanding of the immune landscape relative to radiotherapy across tumor types. Front. Immunol. 14, 1148692 (2023).
Ostrand-Rosenberg, S., Horn, L. A. & Ciavattone, N. G. Radiotherapy both promotes and inhibits myeloid-derived suppressor cell function: novel strategies for preventing the tumor-protective effects of radiotherapy. Front. Oncol. 9, 215 (2019).
Svoronos, N. et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. 7, 72–85 (2017).
Bergenfelz, C. et al. Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE 10, e0127028 (2015).
Larsson, A.-M., Roxå, A., Leandersson, K. & Bergenfelz, C. Impact of systemic therapy on circulating leukocyte populations in patients with metastatic breast cancer. Sci. Rep. 9, 13451 (2019).
Márquez-Garbán, D. C. et al. Antiestrogens in combination with immune checkpoint inhibitors in breast cancer immunotherapy. J. Steroid Biochem. Mol. Biol. 193, 105415 (2019).
Milette, S. et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat. Commun. 10, 5745 (2019).
Qin, C., Wang, J., Du, Y. & Xu, T. Immunosuppressive environment in response to androgen deprivation treatment in prostate cancer. Front. Endocrinol. 13, 1055826 (2022).
Xu, P. et al. Androgen receptor blockade resistance with enzalutamide in prostate cancer results in immunosuppressive alterations in the tumor immune microenvironment. J. Immunother. Cancer 11, e006581 (2023).
Wang, D. et al. IL-1β is an androgen-responsive target in macrophages for immunotherapy of prostate cancer. Adv. Sci. 10, 2206889 (2023).
Lopez-Bujanda, Z. A. et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat. Cancer 2, 803–818 (2021).
Consiglio, C. R., Udartseva, O., Ramsey, K. D., Bush, C. & Gollnick, S. O. Enzalutamide, an androgen receptor antagonist, enhances myeloid cell-mediated immune suppression and tumor progression. Cancer Immunol. Res. 8, 1215–1227 (2020).
Calcinotto, A. et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 (2018).
Pienta, K. J. et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest. New Drugs 31, 760–768 (2013).
Brana, I. et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target. Oncol. 10, 111–123 (2015).
Nywening, T. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016).
Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016).
Haag, G. M. et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer — the PICCASSO phase I trial. Eur. J. Cancer 167, 112–122 (2022).
Johnson, M. et al. ARRY-382 in combination with pembrolizumab in patients with advanced solid tumors: results from a phase 1b/2 study. Clin. Cancer Res. 28, 2517–2526 (2022).
Machiels, J.-P. et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J. Immunother. Cancer 8, e001153 (2020).
Gomez-Roca, C. et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naïve or experienced for immune checkpoint blockade. J. Immunother. Cancer 10, e004076 (2022).
Autio, K. A. et al. Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: a phase I study. Clin. Cancer Res. 26, 5609–5620 (2020).
Dowlati, A. et al. LY3022855, an anti–colony stimulating factor-1 receptor (CSF-1R) monoclonal antibody, in patients with advanced solid tumors refractory to standard therapy: phase 1 dose-escalation trial. Invest. New Drugs 39, 1057–1071 (2021).
Falchook, G. S. et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Invest. New Drugs 39, 1284–1297 (2021).
Schott, A. F. et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin. Cancer Res. 23, 5358–5365 (2017).
Goldstein, L. J. et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 190, 265–275 (2021).
Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med. 26, 878–885 (2020).
Biasci, D. et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl Acad. Sci. USA 117, 28960–28970 (2020).
Proia, T. A. et al. STAT3 antisense oligonucleotide remodels the suppressive tumor microenvironment to enhance immune activation in combination with anti–PD-L1. Clin. Cancer Res. 26, 6335–6349 (2020).
Nishina, T. et al. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: a phase 1 study. BMJ Open 12, e055718 (2022).
Miller, J. S. et al. 965MO GTB-3550 tri-specific killer engager safely activates and delivers IL-15 to NK cells, but not T-cells, in immune suppressed patients with advanced myeloid malignancies, a novel paradigm exportable to solid tumors expressing Her2 or B7H3. Ann. Oncol. 32, S834 (2021).
Lorentzen, C. L. et al. Arginase-1 targeting peptide vaccine in patients with metastatic solid tumors — a phase I trial. Front. Immunol. 13, 1023023 (2022).
Hashimoto, A. et al. Upregulation of C/EBPα inhibits suppressive activity of myeloid cells and potentiates antitumor response in mice and patients with cancer. Clin. Cancer Res. 27, 5961–5978 (2021).
Meyerhardt, J. A. et al. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA 325, 1277–1286 (2021).
James, N. D. et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 13, 549–558 (2012).
Mason, M. D. et al. Adding celecoxib with or without zoledronic acid for hormone-naïve prostate cancer: long-term survival results from an adaptive, multiarm, multistage, platform, randomized controlled trial. J. Clin. Oncol. 35, 1530–1541 (2017).
Iversen, T. Z. et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin. Cancer Res. 20, 221–232 (2014).
Bjoern, J., Iversen, T. Z., Nitschke, N. J., Andersen, M. H. & Svane, I. M. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy 18, 1043–1055 (2016).
Komrokji, R. S. et al. A phase II study to determine the safety and efficacy of the oral inhibitor of indoleamine 2,3-dioxygenase (IDO) enzyme INCB024360 in patients with myelodysplastic syndromes. Clin. Lymphoma Myeloma Leuk. 19, 157–161 (2019).
Powderly, J. D. et al. Epacadostat plus pembrolizumab and chemotherapy for advanced solid tumors: results from the phase I/II ECHO-207/KEYNOTE-723 study. Oncologist 27, 905–e848 (2022).
Johnson, T. S. et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: a first-in-children phase 1 trial.Neuro-Oncology https://doi.org/10.1093/neuonc/noad174 (2023).
Nayak-Kapoor, A. et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J. Immunother. Cancer 6, 61 (2018).
Patel, S. P. et al. A phase 1b/2 study of omaveloxolone in combination with checkpoint inhibitors in patients with unresectable or metastatic melanoma. Ann. Oncol. 28, xi30 (2017).
Califano, J. A. et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 30–38 (2015).
Weed, D. T. et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 39–48 (2015).
Weed, D. T. et al. The reversal of immune exclusion mediated by tadalafil and an anti-tumor vaccine also induces PDL1 upregulation in recurrent head and neck squamous cell carcinoma: interim analysis of a phase I clinical trial. Front. Immunol. 10, 1206 (2019).
Iclozan, C., Antonia, S., Chiappori, A., Chen, D.-T. & Gabrilovich, D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 62, 909–918 (2013).
Tobin, R. P. et al. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int. Immunopharmacol. 63, 282–291 (2018).
Tobin, R. P. et al. Targeting MDSC differentiation using ATRA: a phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin. Cancer Res. 29, 1209–1219 (2023).
Shayan, G. et al. Phase Ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin. Cancer Res. 24, 62–72 (2018).
Yamauchi, Y. et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 198, 777–787 (2018).
Kim, H. R. et al. The ratio of peripheral regulatory T cells to lox-1+ polymorphonuclear myeloid-derived suppressor cells predicts the early response to anti-PD-1 therapy in patients with non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 199, 243–246 (2019).
Si, Y. et al. Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci. Immunol. 4, eaaw9159 (2019).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081 (2017).
Zilionis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334.e10 (2019).
Author information
Authors and Affiliations
Contributions
S.A.L. and V.U. researched data for the article and wrote the manuscript. All authors contributed substantially to the discussion of content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S. Brandau, R. Kiessling and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lasser, S.A., Ozbay Kurt, F.G., Arkhypov, I. et al. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol 21, 147–164 (2024). https://doi.org/10.1038/s41571-023-00846-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00846-y
This article is cited by
-
Current and future immunotherapeutic approaches in pancreatic cancer treatment
Journal of Hematology & Oncology (2024)