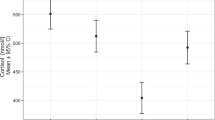
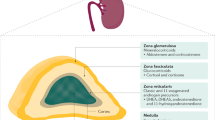
Abstract
The majority of incidentally discovered adrenal tumours are benign adrenocortical adenomas and the prevalence of adrenocortical adenomas is around 1–7% on cross-sectional abdominal imaging. These can be non-functioning adrenal tumours or they can be associated with autonomous cortisol secretion on a spectrum that ranges from rare clinically overt adrenal Cushing syndrome to the much more prevalent mild autonomous cortisol secretion (MACS) without signs of Cushing syndrome. MACS is diagnosed (based on an abnormal overnight dexamethasone suppression test) in 20–50% of patients with adrenal adenomas. MACS is associated with cardiovascular morbidity, frailty, fragility fractures, decreased quality of life and increased mortality. Management of MACS should be individualized based on patient characteristics and includes adrenalectomy or conservative follow-up with treatment of associated comorbidities. Identifying patients with MACS who are most likely to benefit from adrenalectomy is challenging, as adrenalectomy results in improvement of cardiovascular morbidity in some, but not all, patients with MACS. Of note, diagnosis and management of patients with bilateral MACS is especially challenging. Current gaps in MACS clinical practice include a lack of specific biomarkers diagnostic of MACS-related health outcomes and a paucity of clinical trials demonstrating the efficacy of adrenalectomy on comorbidities associated with MACS. In addition, little evidence exists to demonstrate the efficacy and safety of long-term medical therapy in patients with MACS.
Key points
-
Mild autonomous cortisol secretion (MACS) is diagnosed based on the 1 mg overnight dexamethasone test and is found in 20–50% of patients with adrenal adenomas lacking signs and symptoms of Cushing syndrome.
-
Patients with adrenal adenomas show distinct changes in the steroid and global metabolome, which correlate with the degree of cortisol excess across MACS and Cushing syndrome.
-
MACS is associated with an increased likelihood of having cardiovascular risk factors and an increased risk of mortality.
-
Management of MACS must be individualized based on patient characteristics; the options range from adrenalectomy to long-term follow-up and conservative management of comorbidities.
-
Post-operative adrenal insufficiency is seen in around 50% of patients with MACS who undergo unilateral adrenalectomy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fassnacht, M. et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 189, G1–G42 (2023).
Ebbehoj, A. et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 8, 894–902 (2020).
Elhassan, Y. S. et al. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann. Intern. Med. 171, 107–116 (2019).
Prete, A. et al. Cardiometabolic disease burden and steroid excretion in benign adrenal tumors: a cross-sectional multicenter study. Ann. Intern. Med. 175, 325–334 (2022).
Deutschbein, T. et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 10, 499–508 (2022).
Dogra, P. et al. High prevalence of frailty in patients with adrenal adenomas and adrenocortical hormone excess: a cross-sectional multi-centre study with prospective enrolment. Eur. J. Endocrinol. 189, 318–326 (2023).
Lopez, D. et al. ‘Nonfunctional’ adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann. Intern. Med. 165, 533–542 (2016).
Bancos, I. & Prete, A. Approach to the patient with adrenal incidentaloma. J. Clin. Endocrinol. Metab. 106, 3331–3353 (2021).
Jing, Y. et al. Prevalence and characteristics of adrenal tumors in an unselected screening population: a cross-sectional study. Ann. Intern. Med. 175, 1383–1391 (2022).
Bancos, I. et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 8, 773–781 (2020).
Mantero, F. et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 85, 637–644 (2000).
Ahn, S. H. et al. Characteristics of adrenal incidentalomas in a large, prospective computed tomography-based multicenter study: the COAR study in Korea. Yonsei Med J. 59, 501–510 (2018).
Ichijo, T., Ueshiba, H., Nawata, H. & Yanase, T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr. J. 67, 141–152 (2020).
Libe, R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front. Cell Dev. Biol. 3, 45 (2015).
Lacroix, A., Feelders, R. A., Stratakis, C. A. & Nieman, L. K. Cushing’s syndrome. Lancet 386, 913–927 (2015).
Mete, O. et al. Overview of the 2022 WHO classification of adrenal cortical tumors. Endocr. Pathol. 33, 155–196 (2022).
Bertherat, J. et al. Clinical, pathophysiologic, genetic, and therapeutic progress in primary bilateral macronodular adrenal hyperplasia. Endocr. Rev. 44, 567–628 (2023).
Li, D. et al. Determinants of muscle function and health-related quality of life in patients with endogenous hypercortisolism: a cross-sectional study. Eur. J. Endocrinol. 188, 603–612 (2023).
Giordano, R. et al. Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur. J. Endocrinol. 162, 779–785 (2010).
Morelli, V. et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J. Clin. Endocrinol. Metab. 99, 827–834 (2014).
Di Dalmazi, G. et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2, 396–405 (2014).
Arruda, M. et al. The presence of nonfunctioning adrenal incidentalomas increases arterial hypertension frequency and severity, and is associated with cortisol levels after dexamethasone suppression test. J. Hum. Hypertens. 32, 3–11 (2017).
Kim, J. H., Kim, M. J., Lee, J. H., Yoon, J. W. & Shin, C. S. Nonfunctioning adrenal incidentalomas are not clinically silent: a longitudinal cohort study. Endocr. Pract. 26, 1406–1415 (2020).
Khan, U. Nonfunctioning and subclinical cortisol secreting adrenal incidentalomas and their association with metabolic syndrome: a systematic review. Indian J. Endocrinol. Metab. 23, 332–346 (2019).
Araujo-Castro, M. et al. Nonfunctioning adrenal incidentalomas with cortisol post-dexamethasone suppression test >0.9 µg/dL have a higher prevalence of cardiovascular disease than those with values ≤0.9 µg/dL. Endocrine 79, 384–391 (2023).
Araujo-Castro, M. et al. Cardiometabolic profile of non-functioning and autonomous cortisol-secreting adrenal incidentalomas. Is the cardiometabolic risk similar or are there differences? Endocrine 66, 650–659 (2019).
Faillot, S. et al. Genomic classification of benign adrenocortical lesions. Endocr. Relat. Cancer 28, 79–95 (2021).
Di Dalmazi, G. et al. RNA sequencing and somatic mutation status of adrenocortical tumors: novel pathogenetic insights. J. Clin. Endocrinol. Metab. 105, dgaa616 (2020).
Wilmot Roussel, H. et al. Identification of gene expression profiles associated with cortisol secretion in adrenocortical adenomas. J. Clin. Endocrinol. Metab. 98, E1109–E1121 (2013).
Jouinot, A., Armignacco, R. & Assie, G. Genomics of benign adrenocortical tumors. J. Steroid Biochem. Mol. Biol. 193, 105414 (2019).
Bonnet, S. et al. Wnt/beta-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J. Clin. Endocrinol. Metab. 96, E419–E426 (2011).
Juhlin, C. C. et al. What did we learn from the molecular biology of adrenal cortical neoplasia? From histopathology to translational genomics. Endocr. Pathol. 32, 102–133 (2021).
Ronchi, C. L. et al. Genetic landscape of sporadic unilateral adrenocortical adenomas without PRKACA p.Leu206Arg mutation. J. Clin. Endocrinol. Metab. 101, 3526–3538 (2016).
Pitsava, G. & Stratakis, C. A. Genetic alterations in benign adrenal tumors. Biomedicines 10, 1041 (2022).
Ronchi, C. L. cAMP/protein kinase A signalling pathway and adrenocortical adenomas. Curr. Opin. Endocr. Metab. Res. 8, 15–21 (2019).
Little, D. W. III, Dumontet, T., LaPensee, C. R. & Hammer, G. D. Beta-catenin in adrenal zonation and disease. Mol. Cell Endocrinol. 522, 111120 (2021).
Vassiliadi, D. A. & Tsagarakis, S. Diagnosis and management of primary bilateral macronodular adrenal hyperplasia. Endocr. Relat. Cancer 26, R567–R581 (2019).
Bouys, L. et al. Identification of predictive criteria for pathogenic variants of primary bilateral macronodular adrenal hyperplasia (PBMAH) gene ARMC5 in 352 unselected patients. Eur. J. Endocrinol. 187, 123–134 (2022).
Morelli, V. et al. Prevalence and clinical features of armadillo repeat-containing 5 mutations carriers in a single center cohort of patients with bilateral adrenal incidentalomas. Eur. J. Endocrinol. 189, 242–251 (2023).
Lacroix, A., Ndiaye, N., Tremblay, J. & Hamet, P. Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr. Rev. 22, 75–110 (2001).
Reznik, Y. et al. Aberrant adrenal sensitivity to multiple ligands in unilateral incidentaloma with subclinical autonomous cortisol hypersecretion: a prospective clinical study. Clin. Endocrinol. 61, 311–319 (2004).
Perraudin, V. et al. Vasopressin-responsive adrenocortical tumor in a mild Cushing’s syndrome: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 80, 2661–2667 (1995).
Tsagarakis, S. et al. Food-dependent androgen and cortisol secretion by a gastric inhibitory polypeptide-receptor expressive adrenocortical adenoma leading to hirsutism and subclinical Cushing’s syndrome: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 86, 583–589 (2001).
Contesse, V. et al. Abnormal sensitivity of cortisol-producing adrenocortical adenomas to serotonin: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 90, 2843–2850 (2005).
Dall’Asta, C. et al. Assessing the presence of abnormal regulation of cortisol secretion by membrane hormone receptors: in vivo and in vitro studies in patients with functioning and non-functioning adrenal adenoma. Horm. Metab. Res. 36, 578–583 (2004).
Carlson, H. E. Human adrenal cortex hyperfunction due to LH/hCG. Mol. Cell Endocrinol. 269, 46–50 (2007).
Bernichtein, S., Alevizaki, M. & Huhtaniemi, I. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol. Metab. 19, 231–238 (2008).
Vassiliadi, D. A., Ntali, G., Stratigou, T., Adali, M. & Tsagarakis, S. Aberrant cortisol responses to physiological stimuli in patients presenting with bilateral adrenal incidentalomas. Endocrine 40, 437–444 (2011).
Marina, L. V. et al. Luteinizing hormone and insulin resistance in menopausal patients with adrenal incidentalomas: the cause–effect relationship? Clin. Endocrinol. 88, 541–548 (2018).
Feelders, R. A. et al. Luteinizing hormone (LH)-responsive Cushing’s syndrome: the demonstration of LH receptor messenger ribonucleic acid in hyperplastic adrenal cells, which respond to chorionic gonadotropin and serotonin agonists in vitro. J. Clin. Endocrinol. Metab. 88, 230–237 (2003).
Lefebvre, H., Prevost, G. & Louiset, E. Autocrine/paracrine regulatory mechanisms in adrenocortical neoplasms responsible for primary adrenal hypercorticism. Eur. J. Endocrinol. 169, R115–R138 (2013).
Lefebvre, H. et al. Paracrine control of steroidogenesis by serotonin in adrenocortical neoplasms. Mol. Cell Endocrinol. 408, 198–204 (2015).
Lefebvre, H., Duparc, C., Prevost, G., Bertherat, J. & Louiset, E. Cell-to-cell communication in bilateral macronodular adrenal hyperplasia causing hypercortisolism. Front. Endocrinol. 6, 34 (2015).
Reincke, M., Fassnacht, M., Vath, S., Mora, P. & Allolio, B. Adrenal incidentalomas: a manifestation of the metabolic syndrome? Endocr. Res. 22, 757–761 (1996).
Muscogiuri, G. et al. The size of adrenal incidentalomas correlates with insulin resistance. Is there a cause–effect relationship? Clin. Endocrinol. 74, 300–305 (2011).
Abdellatif, A. B., Fernandes-Rosa, F. L., Boulkroun, S. & Zennaro, M. C. Vascular and hormonal interactions in the adrenal gland. Front. Endocrinol. 13, 995228 (2022).
Higgs, J. A. et al. Pathophysiological link between insulin resistance and adrenal incidentalomas. Int. J. Mol. Sci. 23, 4340 (2022).
Altieri, B. et al. Adrenocortical tumors and insulin resistance: what is the first step? Int. J. Cancer 138, 2785–2794 (2016).
Sydney, G. I., Ioakim, K. J. & Paschou, S. A. Insulin resistance and adrenal incidentalomas: a bidirectional relationship. Maturitas 121, 1–6 (2019).
Arlt, W. et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 96, 3775–3784 (2011).
Hines, J. M. et al. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin. Chem. 63, 1824–1835 (2017).
Kerkhofs, T. M., Kerstens, M. N., Kema, I. P., Willems, T. P. & Haak, H. R. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm. Cancer 6, 168–175 (2015).
Arlt, W. et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2, e93136 (2017).
Kikuchi, E. et al. Urinary steroid profile in adrenocortical tumors. Biomed. Pharmacother. 54, 194s–197s (2000).
Homoki, J., Holl, R. & Teller, W. M. [Urinary steroid profile in Cushing syndrome and in tumors of the adrenal cortex]. Klin. Wochenschr. 65, 719–726 (1987).
Kotlowska, A., Puzyn, T., Sworczak, K., Stepnowski, P. & Szefer, P. Metabolomic biomarkers in urine of Cushing’s syndrome patients. Int. J. Mol. Sci. 18, 294 (2017).
Tiu, S. C. et al. Use of urinary steroid profiling for diagnosing and monitoring adrenocortical tumours. Hong Kong Med. J. 15, 463–470 (2009).
Velikanova, L. I. et al. Different types of urinary steroid profiling obtained by high-performance liquid chromatography and gas chromatography–mass spectrometry in patients with adrenocortical carcinoma. Horm. Cancer 7, 327–335 (2016).
Brossaud, J., Ducint, D. & Corcuff, J. B. Urinary glucocorticoid metabolites: biomarkers to classify adrenal incidentalomas? Clin. Endocrinol. 84, 236–243 (2016).
Maser-Gluth, C., Reincke, M., Allolio, B. & Schulze, E. Metabolism of glucocorticoids and mineralocorticoids in patients with adrenal incidentalomas. Eur. J. Clin. Invest. 30, 83–86 (2000).
Araujo-Castro, M. et al. Is the 1 mg-dexamethasone suppression test a precise marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas? Endocrine 82, 161–170 (2023).
Berke, K. et al. Plasma steroid profiling in patients with adrenal incidentaloma. J. Clin. Endocrinol. Metab. 107, e1181–e1192 (2022).
Hana, V. Jr et al. Serum steroid profiling in Cushing’s syndrome patients. J. Steroid Biochem. Mol. Biol. 192, 105410 (2019).
Masjkur, J. et al. Plasma steroid profiles in subclinical compared with overt adrenal Cushing syndrome. J. Clin. Endocrinol. Metab. 104, 4331–4340 (2019).
Eisenhofer, G. et al. Plasma steroid metabolome profiling for diagnosis and subtyping patients with Cushing syndrome. Clin. Chem. 64, 586–596 (2018).
Di Dalmazi, G. et al. Steroid profiling by LC–MS/MS in nonsecreting and subclinical cortisol-secreting adrenocortical adenomas. J. Clin. Endocrinol. Metab. 100, 3529–3538 (2015).
Ueshiba, H., Segawa, M., Hayashi, T., Miyachi, Y. & Irie, M. Serum profiles of steroid hormones in patients with Cushing’s syndrome determined by a new HPLC/RIA method. Clin. Chem. 37, 1329–1333 (1991).
Di Dalmazi, G. et al. The steroid profile of adrenal incidentalomas: subtyping subjects with high cardiovascular risk. J. Clin. Endocrinol. Metab. 104, 5519–5528 (2019).
Ku, E. J. et al. Metabolic subtyping of adrenal tumors: prospective multi-center cohort study in Korea. Endocrinol. Metab. 36, 1131–1141 (2021).
Huayllas, M. K. P. et al. Steroidogenesis in patients with adrenal incidentalomas: extended steroid profile measured by liquid chromatography–mass spectrometry after ACTH stimulation and dexamethasone suppression. Clin. Endocrinol. 95, 29–40 (2021).
Constantinescu, G. et al. Glucocorticoid excess in patients with pheochromocytoma compared with paraganglioma and other forms of hypertension. J. Clin. Endocrinol. Metab. 105, e3374–e3383 (2020).
Dennedy, M. C. et al. Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J. Clin. Endocrinol. Metab. 102, 786–792 (2017).
Hana, V. et al. Novel GC–MS/MS technique reveals a complex steroid fingerprint of subclinical hypercortisolism in adrenal incidentalomas. J. Clin. Endocrinol. Metab. 104, 3545–3556 (2019).
Hannah-Shmouni, F. et al. Mass spectrometry-based steroid profiling in primary bilateral macronodular adrenocortical hyperplasia. Endocr. Relat. Cancer 27, 403–413 (2020).
Teuber, J. P. et al. Intratumoral steroid profiling of adrenal cortisol-producing adenomas by liquid chromatography–mass spectrometry. J. Steroid Biochem. Mol. Biol. 212, 105924 (2021).
Murakami, M. et al. In situ metabolomics of cortisol-producing adenomas. Clin. Chem. 69, 149–159 (2023).
Bassett, M. H. et al. Expression profiles for steroidogenic enzymes in adrenocortical disease. J. Clin. Endocrinol. Metab. 90, 5446–5455 (2005).
Cao, C. et al. Increased expression of CYP17 and CYP11B1 in subclinical Cushing’s syndrome due to adrenal adenomas. Int. J. Urol. 18, 691–696 (2011).
Di Dalmazi, G. et al. Cortisol-related metabolic alterations assessed by mass spectrometry assay in patients with Cushing’s syndrome. Eur. J. Endocrinol. 177, 227–237 (2017).
Erlic, Z. et al. Targeted metabolomics as a tool in discriminating endocrine from primary hypertension. J. Clin. Endocrinol. Metab. 106, 1111–1128 (2021).
Vega-Beyhart, A. et al. Endogenous cortisol excess confers a unique lipid signature and metabolic network. J. Mol. Med. 99, 1085–1099 (2021).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 1859, 1558–1572 (2017).
Supale, S., Li, N., Brun, T. & Maechler, P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol. Metab. 23, 477–487 (2012).
Ren, J., Pulakat, L., Whaley-Connell, A. & Sowers, J. R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. 88, 993–1001 (2010).
Funai, K. et al. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes 65, 358–370 (2016).
Funai, K. et al. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J. Clin. Invest. 123, 1229–1240 (2013).
Storlien, L. H. et al. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40, 280–289 (1991).
Chang, W., Hatch, G. M., Wang, Y., Yu, F. & Wang, M. The relationship between phospholipids and insulin resistance: from clinical to experimental studies. J. Cell. Mol. Med. 23, 702–710 (2019).
Listenberger, L. L. et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA 100, 3077–3082 (2003).
Guo, Y. et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453, 657–661 (2008).
Meikle, P. J. et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE 8, e74341 (2013).
Semba, R. D. et al. Altered plasma amino acids and lipids associated with abnormal glucose metabolism and insulin resistance in older adults. J. Clin. Endocrinol. Metab. 103, 3331–3339 (2018).
Morze, J. et al. Metabolomics and type 2 diabetes risk: an updated systematic review and meta-analysis of prospective cohort studies. Diabetes Care 45, 1013–1024 (2022).
Tan, S. T., Ramesh, T., Toh, X. R. & Nguyen, L. N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 80, 101068 (2020).
Rodriguez-Cuenca, S., Pellegrinelli, V., Campbell, M., Oresic, M. & Vidal-Puig, A. Sphingolipids and glycerophospholipids — The ‘Ying and Yang’ of lipotoxicity in metabolic diseases. Prog. Lipid Res. 66, 14–29 (2017).
Green, C. D., Maceyka, M., Cowart, L. A. & Spiegel, S. Sphingolipids in metabolic disease: the good, the bad, and the unknown. Cell Metab. 33, 1293–1306 (2021).
Yin, X. et al. Lipidomic profiling identifies signatures of metabolic risk. eBioMedicine 51, 102520 (2020).
Markgraf, D. F., Al-Hasani, H. & Lehr, S. Lipidomics — reshaping the analysis and perception of type 2 diabetes. Int. J. Mol. Sci. 17, 1841 (2016).
Razquin, C. et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care 41, 2617–2624 (2018).
Liu, P. et al. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 247, 117443 (2020).
Matsumoto, T., Kobayashi, T. & Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 14, 3209–3220 (2007).
Mihalik, S. J. et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 18, 1695–1700 (2010).
Guasch-Ferre, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
Yu, E. et al. Changes in arginine are inversely associated with type 2 diabetes: a case–cohort study in the PREDIMED trial. Diabetes Obes. Metab. 21, 397–401 (2019).
Chen, S. et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci. Rep. 9, 7010 (2019).
Gunther, S. H. et al. Serum acylcarnitines and amino acids and risk of type 2 diabetes in a multiethnic Asian population. BMJ Open Diabetes Res. Care 8, e001315 (2020).
Pitocco, D. et al. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 7, 15–25 (2010).
Montagnani, M. & Quon, M. J. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes. Metab. 2, 285–292 (2000).
DiNicolantonio, J. J., McCarty, M. F. & James, H. O. K. Role of dietary histidine in the prevention of obesity and metabolic syndrome. Open Heart 5, e000676 (2018).
Toyoshima, K. et al. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE 12, e0185206 (2017).
Lu, Y. et al. Systemic and metabolic signature of sarcopenia in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 75, 309–317 (2020).
Le Couteur, D. G. et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1805–1810 (2020).
Bjelakovic, G. et al. Metabolic correlations of glucocorticoids and polyamines in inflammation and apoptosis. Amino Acids 39, 29–43 (2010).
Carafone, L. E. et al. Diagnostic accuracy of dehydroepiandrosterone sulfate and corticotropin in autonomous cortisol secretion. Biomedicines https://doi.org/10.3390/biomedicines9070741 (2021).
Genere, N. et al. Interpretation of abnormal dexamethasone suppression test is enhanced with use of synchronous free cortisol assessment. J. Clin. Endocrinol. Metab. 107, e1221–e1230 (2022).
Fassnacht, M. et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016).
Kjellbom, A., Lindgren, O., Puvaneswaralingam, S., Londahl, M. & Olsen, H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas : a cohort study. Ann. Intern. Med. 174, 1041–1049 (2021).
Ueland, G. A. et al. Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur. J. Endocrinol. 176, 705–713 (2017).
Atkins, J. S. et al. Serum cortisol assay performance following the 1 mg overnight dexamethasone suppression test. Ann. Clin. Biochem. 60, 386–395 (2023).
Issa, B. G. et al. The utility of salivary cortisone in the overnight dexamethasone suppression test in adrenal incidentalomas. J. Clin. Endocrinol. Metab. 108, e937–e943 (2023).
Patrova, J., Mannheimer, B., Lindh, J. D. & Falhammar, H. Mortality in patients with nonfunctional adrenal tumors. JAMA Intern. Med. 183, 832–838 (2023).
Zhang, C. D. et al. Cardiometabolic outcomes and mortality in patients with adrenal adenomas in a population-based setting. J. Clin. Endocrinol. Metab. 106, 3320–3330 (2021).
Di Dalmazi, G. et al. Prevalence and incidence of atrial fibrillation in a large cohort of adrenal incidentalomas: a long-term study. J. Clin. Endocrinol. Metab. 105, dgaa270 (2020).
Pelsma, I. C. M. et al. Comorbidities in mild autonomous cortisol secretion and the effect of treatment: systematic review and meta-analysis. Eur. J. Endocrinol. 189, S88–S101 (2023).
Singh, S., Atkinson, E. J., Achenbach, S. J., LeBrasseur, N. & Bancos, I. Frailty in patients with mild autonomous cortisol secretion is higher than in patients with nonfunctioning adrenal tumors. J. Clin. Endocrinol. Metab. 105, e3307–e3315 (2020).
Delivanis, D. A. et al. Abnormal body composition in patients with adrenal adenomas. Eur. J. Endocrinol. 185, 653–662 (2021).
Petramala, L. et al. Cardiovascular and metabolic risk factors in patients with subclinical Cushing. Endocrine 70, 150–163 (2020).
Park, J., De Luca, A., Dutton, H., Malcolm, J. C. & Doyle, M. A. Cardiovascular outcomes in autonomous cortisol secretion and nonfunctioning adrenal adenoma: a systematic review. J. Endocr. Soc. 3, 996–1008 (2019).
Favero, V. et al. The degree of cortisol secretion is associated with diabetes mellitus and hypertension in patients with nonfunctioning adrenal tumors. Cardiovasc. Diabetol. 22, 102 (2023).
Kjellbom, A., Lindgren, O., Danielsson, M., Olsen, H. & Londahl, M. Mortality not increased in patients with nonfunctional adrenal adenomas: a matched cohort study. J. Clin. Endocrinol. Metab. 108, e536–e541 (2023).
Li, D. et al. Risk of bone fractures after the diagnosis of adrenal adenomas: a population-based cohort study. Eur. J. Endocrinol. 184, 597–606 (2021).
Zavatta, G. et al. Mild autonomous cortisol secretion in adrenal incidentalomas and risk of fragility fractures: a large cross-sectional study. Eur. J. Endocrinol. 188, 343–352 (2023).
Hans, D. et al. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J. Clin. Densitom. 14, 302–312 (2011).
Eller-Vainicher, C. et al. Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J. Bone Miner. Res. 27, 2223–2230 (2012).
Kim, B. J. et al. The association of cortisol and adrenal androgen with trabecular bone score in patients with adrenal incidentaloma with and without autonomous cortisol secretion. Osteoporos. Int. 29, 2299–2307 (2018).
Yano, C. et al. Coexistence of bone and vascular disturbances in patients with endogenous glucocorticoid excess. Bone Rep. 17, 101610 (2022).
Liu, M. S. et al. Impaired cognitive function in patients with autonomous cortisol secretion in adrenal incidentalomas. J. Clin. Endocrinol. Metab. 108, 633–641 (2023).
Sojat, A. S. et al. Depression: another cortisol-related comorbidity in patients with adrenal incidentalomas and (possible) autonomous cortisol secretion. J. Endocrinol. Invest. 44, 1935–1945 (2021).
Morelli, V. et al. Mental health in patients with adrenal incidentalomas: is there a relation with different degrees of cortisol secretion? J. Clin. Endocrinol. Metab. 106, e130–e139 (2021).
Li, D. et al. Risk of dementia and psychiatric or sleep disorders after diagnosis of adrenal adenomas: a population-based cohort study. Eur. J. Endocrinol. 189, 429–437 (2023).
Bancos, I. et al. Therapy of endocrine disease: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur. J. Endocrinol. 175, R283–R295 (2016).
Morelli, V. et al. Adrenalectomy improves blood pressure and metabolic control in patients with possible autonomous cortisol secretion: results of a RCT. Front. Endocrinol. 13, 898084 (2022).
Di Dalmazi, G., Berr, C. M., Fassnacht, M., Beuschlein, F. & Reincke, M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J. Clin. Endocrinol. Metab. 99, 2637–2645 (2014).
DeLozier, O. M. et al. Selective glucocorticoid replacement following unilateral adrenalectomy for hypercortisolism and primary aldosteronism. J. Clin. Endocrinol. Metab. 107, e538–e547 (2022).
Hurtado, M. D., Cortes, T., Natt, N., Young, W. F. Jr & Bancos, I. Extensive clinical experience: hypothalamic–pituitary–adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin. Endocrinol. 89, 721–733 (2018).
Zhang, C. D. et al. Glucocorticoid withdrawal syndrome following surgical remission of endogenous hypercortisolism: a longitudinal observational study. Eur. J. Endocrinol. 188, 592–602 (2023).
Herndon, J. et al. The effect of curative treatment on hyperglycemia in patients with Cushing syndrome. J. Endocr. Soc. 6, bvab169 (2022).
Chabre, O., Young, J., Caron, P. & Tabarin, A. Letter to the editor: ‘long-term outcome of primary bilateral macronodular adrenocortical hyperplasia after unilateral adrenalectomy’. J. Clin. Endocrinol. Metab. 105, e920–e921 (2020).
Debillon, E. et al. Unilateral adrenalectomy as a first-line treatment of Cushing’s syndrome in patients with primary bilateral macronodular adrenal hyperplasia. J. Clin. Endocrinol. Metab. 100, 4417–4424 (2015).
Osswald, A. et al. Long-term outcome of primary bilateral macronodular adrenocortical hyperplasia after unilateral adrenalectomy. J. Clin. Endocrinol. Metab. 104, 2985–2993 (2019).
Ueland, G. A. et al. Adrenal venous sampling for assessment of autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 103, 4553–4560 (2018).
Johnson, P. C. et al. Adrenal venous sampling for lateralization of cortisol hypersecretion in patients with bilateral adrenal masses. Clin. Endocrinol. 98, 177–189 (2023).
Belokovskaya, R. et al. Mifepristone treatment for mild autonomous cortisol secretion due to adrenal adenomas: a pilot study. Endocr. Pract. 25, 846–853 (2019).
Oda, S. et al. An open-label phase I/IIa clinical trial of 11beta-HSD1 inhibitor for Cushing’s syndrome and autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 106, e3865–e3880 (2021).
Debono, M. et al. Resetting the abnormal circadian cortisol rhythm in adrenal incidentaloma patients with mild autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 102, 3461–3469 (2017).
Acknowledgements
A.P. receives support from the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (grant reference number NIHR203326) and the European Union’s Horizon 2022 Research and Innovation Programme (call HORIZON-HLTH-2022-TOOL-11; project number 101095407). The views expressed are those of the author (authors) and not necessarily those of the NIHR or the Department of Health and Social Care UK. I.B. is partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Aging (NIA) of the National Institutes of Health (NIH) USA under awards K23DK121888, R03DK132121 and R03AG71934 (to I.B). The views expressed are those of the authors and not necessarily those of the National Institutes of Health. The authors thank W. Young (Mayo Clinic, Rochester, MN, USA), W. Arlt (Imperial College London, UK), R. Sandooja (Mayo Clinic, Rochester, MN, USA), L. Rahimi (Mayo Clinic, Rochester, MN, USA), C. Ronchi (University of Birmingham, UK), O. Suntornlohanakul (University of Birmingham, UK) and G. Di Dalmazi (University of Bologna, Italy) for their critical review of the manuscript and useful suggestions.
Author information
Authors and Affiliations
Contributions
A.P. and I.B. contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.P. received research funding and consultancy fees from Recordati, Diurnal and HRA Pharma. I.B. reports consulting, advisory board or data safety monitoring board participation fees (to institution) from Diurnal, Neurocrine, Spruce, Adrenas, Recordati, Corcept, Sparrow, and HRA Pharma, NovoNordisk, AstraZeneca, Xeris outside this work. I.B. received research funding (to institution) from Recordati and HRA Pharma for investigator-initiated awards.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Filippo Ceccato, Ljiljana Marina and Antoine Tabarin for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prete, A., Bancos, I. Mild autonomous cortisol secretion: pathophysiology, comorbidities and management approaches. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00984-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-024-00984-y