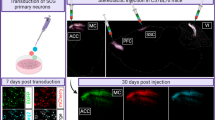
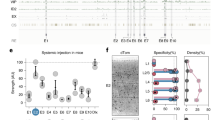
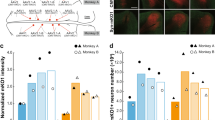
Abstract
Recombinant viruses are the workhorse of modern neuroscience. Whether one would like to understand a neuron’s morphology, natural activity patterns, molecular composition, connectivity or behavioural and physiologic function, most studies begin with the injection of an engineered virus, often an adeno-associated virus or herpes simplex virus, among many other types. Recombinant viruses currently enable some combination of cell type-specific, circuit-selective, activity-dependent and spatiotemporally resolved transgene expression. Viruses are now used routinely to study the molecular and cellular functions of a gene within an identified cell type in the brain, and enable the application of optogenetics, chemogenetics, calcium imaging and related approaches. These advantageous properties of engineered viruses thus enable characterization of neuronal function at unprecedented resolution. However, each virus has specific advantages and disadvantages, which makes viral tool selection paramount for properly designing and executing experiments within the central nervous system. In the current Review, we discuss the key principles and uses of engineered viruses and highlight innovations that are needed moving forward.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Baltimore, D. Expression of animal virus genomes. Bacteriol. Rev. 35, 235–241 (1971).
Lefkowitz, E. J. et al. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 46, D708–D717 (2018).
Flint, S. J., Racaniello, V. R., Rall, G. G., Skalka, A. M. & Enquist, L. W. Principles of Virology 4th edn Vol. 4 (ASM, 2015).
Seo, J. W. et al. Positron emission tomography imaging of novel AAV capsids maps rapid brain accumulation. Nat. Commun. 11, 2102 (2020).
Knowland, D. et al. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170, 284–297.e18 (2017).
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M. & Callaway, E. M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA 107, 21848–21853 (2010). This paper describes the first use of monosynaptic retrograde tracing from a genetically defined post-synaptic population, using a rabies-based system.
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008). This paper applies the FLEX switch to AAVs, in tandem with Cre-driver lines, to achieve highly cell-selective yet robust gene expression.
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014). This paper, building on previous advances in transgenic reporter lines, describes a compact viral method for intersectional genetic cell type targeting.
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Kay, M. A., Glorioso, J. C. & Naldini, L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 7, 33–40 (2001).
Osakada, F. & Callaway, E. M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601 (2013).
Jacobs, A., Breakefield, X. O. & Fraefel, C. HSV-1-based vectors for gene therapy of neurological diseases and brain tumors: part II. Vector systems and applications. Neoplasia 1, 402–416 (1999).
Ekstrand, M. I., Enquist, L. W. & Pomeranz, L. E. The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol. Med. 14, 134–140 (2008).
Zetsche, B., Volz, S. E. & Zhang, F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol. 33, 139–142 (2015).
Truong, D. J. et al. Development of an intein-mediated split-Cas9 system for gene therapy. Nucleic Acids Res. 43, 6450–6458 (2015).
Allen, W. E. et al. Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 94, 891–907.e6 (2017).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016). This paper uses AAV capsid variation to achieve broad gene expression patterns after a peripheral viral injection.
Challis, C. et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 23, 327–336 (2020).
Bedbrook, C. N., Deverman, B. E. & Gradinaru, V. Viral strategies for targeting the central and peripheral nervous systems. Annu. Rev. Neurosci. 41, 323–348 (2018).
Szablowski, J. O., Lee-Gosselin, A., Lue, B., Malounda, D. & Shapiro, M. G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2, 475–484 (2018).
Pomeranz, L. E. et al. Gene expression profiling with Cre-conditional pseudorabies virus reveals a subset of midbrain neurons that participate in reward circuitry. J. Neurosci. 37, 4128–4144 (2017).
Pomeranz, L. E., Reynolds, A. E. & Hengartner, C. J. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69, 462–500 (2005).
Junyent, F. & Kremer, E. J. CAV-2—why a canine virus is a neurobiologist’s best friend. Curr. Opin. Pharmacol. 24, 86–93 (2015).
Ekstrand, M. I. et al. Molecular profiling of neurons based on connectivity. Cell 157, 1230–1242 (2014).
Nectow, A. R., Ekstrand, M. I. & Friedman, J. M. Molecular characterization of neuronal cell types based on patterns of projection with Retro-TRAP. Nat. Protoc. 10, 1319–1327 (2015).
Smith, B. N. et al. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl Acad. Sci. USA 97, 9264–9269 (2000).
Li, S. J., Vaughan, A., Sturgill, J. F. & Kepecs, A. A viral receptor complementation strategy to overcome CAV-2 tropism for efficient retrograde targeting of neurons. Neuron 98, 905–917.e5 (2018).
Murlidharan, G., Samulski, R. J. & Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 7, 76 (2014).
Watakabe, A. et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res. 93, 144–157 (2015).
Burger, C. et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 10, 302–317 (2004).
Juttner, J. et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 22, 1345–1356 (2019).
Callaway, E. M. & Luo, L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 35, 8979–8985 (2015).
Sakurai, K. et al. Capturing and manipulating activated neuronal ensembles with CANE delineates a hypothalamic social-fear circuit. Neuron 92, 739–753 (2016).
McCarty, D. M., Monahan, P. E. & Samulski, R. J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8, 1248–1254 (2001).
Poulin, J. F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Fenno, L. E. et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107, 836–853.e11 (2020).
Dias, C. et al. β-Catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516, 51–55 (2014).
Tsai, H. C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
von Jonquieres, G. et al. Recombinant human myelin-associated glycoprotein promoter drives selective AAV-mediated transgene expression in oligodendrocytes. Front. Mol. Neurosci. 9, 13 (2016).
Nagai, J. et al. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 177, 1280–1292.e20 (2019).
Korbelin, J. et al. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med. 8, 609–625 (2016).
Marchio, S., Sidman, R. L., Arap, W. & Pasqualini, R. Brain endothelial cell-targeted gene therapy of neurovascular disorders. EMBO Mol. Med. 8, 592–594 (2016).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Nectow, A. R. et al. Rapid molecular profiling of defined cell types using viral TRAP. Cell Rep. 19, 655–667 (2017).
Murugan, M. et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e16 (2017).
Ye, L. et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell 165, 1776–1788 (2016).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits. Neuron 57, 634–660 (2008). This paper, an update on the original landmark review, builds on the cutting-edge genetic strategies used to study neural circuits today.
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018).
He, T. et al. The influence of murine genetic background in adeno-associated virus transduction of the mouse brain. Hum. Gene Ther. Clin. Dev. 30, 169–181 (2019).
McCarthy, K. M., Tank, D. W. & Enquist, L. W. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog. 5, e1000640 (2009).
Xiong, W. et al. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl Acad. Sci. USA 116, 5785–5794 (2019).
Beier, K. T. et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl Acad. Sci. USA 108, 15414–15419 (2011).
Lo, L. & Anderson, D. J. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950 (2011).
Kelly, R. M. & Strick, P. L. Rabies as a transneuronal tracer of circuits in the central nervous system. J. Neurosci. Methods 103, 63–71 (2000).
Chatterjee, S. et al. Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat. Neurosci. 21, 638–646 (2018).
Reardon, T. R. et al. Rabies virus CVS-N2c(DeltaG) strain enhances retrograde synaptic transfer and neuronal viability. Neuron 89, 711–724 (2016).
Oyibo, H. K., Znamenskiy, P., Oviedo, H. V., Enquist, L. W. & Zador, A. M. Long-term Cre-mediated retrograde tagging of neurons using a novel recombinant pseudorabies virus. Front. Neuroanat. 8, 86 (2014).
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016). This paper uses viral engineering to identify an effective retrograde-tracing AAV.
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017). This paper identifies different pre-existing AAV serotypes that are capable of anterograde tracing.
Zingg, B., Peng, B., Huang, J., Tao, H. W. & Zhang, L. I. Synaptic specificity and application of anterograde transsynaptic AAV for probing neural circuitry. J. Neurosci. 40, 3250–3267 (2020).
Pena, C. J. et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188 (2017).
Hultman, R. et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell 173, 166–180.e14 (2018).
Kaspar, B. K. et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl Acad. Sci. USA 99, 2320–2325 (2002).
Hayat, H. et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 6, eaaz4232 (2020).
Mayford, M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996).
Nathanson, J. L. et al. Short promoters in viral vectors drive selective expression in mammalian inhibitory neurons, but do not restrict activity to specific inhibitory cell-types. Front. Neural Circuits 3, 19 (2009).
Mehta, P. et al. Functional access to neuron subclasses in rodent and primate forebrain. Cell Rep. 26, 2818–2832.e8 (2019).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl Acad. Sci. USA 101, 18206–18211 (2004).
Dimidschstein, J. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 19, 1743–1749 (2016).
Stamatakis, A. M. et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80, 1039–1053 (2013).
Allen, W. E. et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155 (2017).
Lee, D., Hyun, J. H., Jung, K., Hannan, P. & Kwon, H. B. A calcium- and light-gated switch to induce gene expression in activated neurons. Nat. Biotechnol. 35, 858–863 (2017).
Wang, W. et al. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol. 35, 864–871 (2017).
Kim, C. K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 18, 222–235 (2017).
Kim, E. J. et al. Extraction of distinct neuronal cell types from within a genetically continuous population. Neuron 107, 274–282.e6 (2020).
Wickersham, I. R., Finke, S., Conzelmann, K. K. & Callaway, E. M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49 (2007). This paper describes restricted retrograde tracing with a modified RbV variant.
Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Schwarz, L. A. et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Banfield, B. W., Kaufman, J. D., Randall, J. A. & Pickard, G. E. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J. Virol. 77, 10106–10112 (2003).
Falkner, A. L. et al. Hierarchical representations of aggression in a hypothalamic–midbrain circuit. Neuron 106, 637–648.e6 (2020).
Schneeberger, M. et al. Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685.e12 (2019).
Beier, K. T. et al. Topological organization of ventral tegmental area connectivity revealed by viral-genetic dissection of input–output relations. Cell Rep. 26, 159–167.e6 (2019).
Chen, X. et al. High-throughput mapping of long-range neuronal projection using in situ sequencing. Cell 179, 772–786.e19 (2019).
Kebschull, J. M. & Zador, A. M. Cellular barcoding: lineage tracing, screening and beyond. Nat. Methods 15, 871–879 (2018).
Kebschull, J. M. et al. High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987 (2016). This paper uses a modified Sindbis virus to profile projection neurons using sequencing, in place of classical imaging approaches.
Lerner, T. N., Ye, L. & Deisseroth, K. Communication in neural circuits: tools, opportunities, and challenges. Cell 164, 1136–1150 (2016).
Kim, D. W. et al. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179, 713–728.e17 (2019).
Yim, Y. Y., Teague, C. D. & Nestler, E. J. In vivo locus-specific neuroepigenome editing. Nat. Rev. Neurosci. 21, 471–484 (2020).
Lorsch, Z. S. et al. Stress resilience is promoted by a Zfp189-driven transcriptional network in prefrontal cortex. Nat. Neurosci. 22, 1413–1423 (2019). This paper is a recent example of the use of viral vectors to modify a single gene locus, through the expression of CRISPR tools to effect locus-specific epigenome editing, in a targeted brain region and to study the downstream molecular, cellular and behavioural consequences.
Labonte, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017).
Bagot, R. C. et al. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron 90, 969–983 (2016).
Palfi, S. et al. Long-term follow-up of a phase I/II study of ProSavin, a lentiviral vector gene therapy for Parkinson’s disease. Hum. Gene Ther. Clin. Dev. 29, 148–155 (2018).
Hitti, F. L., Yang, A. I., Gonzalez-Alegre, P. & Baltuch, G. H. Human gene therapy approaches for the treatment of Parkinson’s disease: an overview of current and completed clinical trials. Parkinsonism Relat. Disord. 66, 16–24 (2019).
Kaplitt, M. G. Gene-targeting approaches for movement disorders: recent advances. Curr. Opin. Neurol. 32, 566–570 (2019).
Ogden, P. J., Kelsic, E. D., Sinai, S. & Church, G. M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 366, 1139–1143 (2019). This paper uses machine learning to guide AAV capsid design.
Bru, T., Salinas, S. & Kremer, E. J. An update on canine adenovirus type 2 and its vectors. Viruses 2, 2134–2153 (2010).
Lundstrom, K. Viral vectors in gene therapy. Diseases 6, 42 (2018).
Dolan, A., Jamieson, F. E., Cunningham, C., Barnett, B. C. & McGeoch, D. J. The genome sequence of herpes simplex virus type 2. J. Virol. 72, 2010–2021 (1998).
McGeoch, D. J. et al. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69, 1531–1574 (1988).
Morissette, G. & Flamand, L. Herpesviruses and chromosomal integration. J. Virol. 84, 12100–12109 (2010).
Neve, R. L., Neve, K. A., Nestler, E. J. & Carlezon, W. A. Jr. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques 39, 381–391 (2005).
Smith, G. A. & Enquist, L. W. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl Acad. Sci. USA 97, 4873–4878 (2000).
Klupp, B. G., Hengartner, C. J., Mettenleiter, T. C. & Enquist, L. W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 78, 424–440 (2004).
Srivastava, A., Lusby, E. W. & Berns, K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45, 555–564 (1983).
Deyle, D. R. & Russell, D. W. Adeno-associated virus vector integration. Curr. Opin. Mol. Ther. 11, 442–447 (2009).
McCarty, D. M., Young, S. M. Jr. & Samulski, R. J. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 38, 819–845 (2004).
Muesing, M. A. et al. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature 313, 450–458 (1985).
Parr-Brownlie, L. C. et al. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front. Mol. Neurosci. 8, 14 (2015).
Blomer, U. et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71, 6641–6649 (1997).
Hioki, H. et al. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 14, 872–882 (2007).
Conzelmann, K. K., Cox, J. H., Schneider, L. G. & Thiel, H. J. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175, 485–499 (1990).
Walker, P. J. et al. Evolution of genome size and complexity in the Rhabdoviridae. PLoS Pathog. 11, e1004664 (2015).
Dietzgen, R. G., Kondo, H., Goodin, M. M., Kurath, G. & Vasilakis, N. The family Rhabdoviridae: mono- and bipartite negative-sense RNA viruses with diverse genome organization and common evolutionary origins. Virus Res. 227, 158–170 (2017).
van den Pol, A. N. et al. Viral strategies for studying the brain, including a replication-restricted self-amplifying δG vesicular stomatis virus that rapidly expresses transgenes in brain and can generate a multicolor golgi-like expression. J. Comp. Neurol. 516, 456–481 (2009).
Kebschull, J. M., Garcia da Silva, P. & Zador, A. M. A new defective helper RNA to produce recombinant Sindbis virus that infects neurons but does not propagate. Front. Neuroanat. 10, 56 (2016).
Hahn, C. S., Hahn, Y. S., Braciale, T. J. & Rice, C. M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc. Natl Acad. Sci. USA 89, 2679–2683 (1992).
Ehrengruber, M. U. Alphaviral gene transfer in neurobiology. Brain Res. Bull. 59, 13–22 (2002).
Lustig, S. et al. Molecular basis of Sindbis virus neurovirulence in mice. J. Virol. 62, 2329–2336 (1988).
Bredenbeek, P. J., Frolov, I., Rice, C. M. & Schlesinger, S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J. Virol. 67, 6439–6446 (1993).
Papez, J. W. A proposed mechanism of emotion. 1937. J. Neuropsychiatry Clin. Neurosci. 7, 103–112 (1995). This paper is the earliest to use a viral approach for neural circuit mapping.
Card, J. P. et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13, 2515–2539 (1993).
Le Gal La Salle, G. et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science 259, 988–990 (1993).
Akli, S. et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 3, 224–228 (1993).
Davidson, B. L., Allen, E. D., Kozarsky, K. F., Wilson, J. M. & Roessler, B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 3, 219–223 (1993).
Kaplitt, M. G. et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 8, 148–154 (1994).
Neve, R. L., Howe, J. R., Hong, S. & Kalb, R. G. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience 79, 435–447 (1997).
Carlezon, W. A. Jr et al. Sensitization to morphine induced by viral-mediated gene transfer. Science 277, 812–814 (1997).
Song, S. et al. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J. Neurochem. 68, 1792–1803 (1997).
Carlezon, W. A. Jr. et al. Regulation of cocaine reward by CREB. Science 282, 2272–2275 (1998).
Kelz, M. B. et al. Expression of the transcription factor δFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276 (1999).
Bolanos, C. A. et al. Phospholipase Cγ in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J. Neurosci. 23, 7569–7576 (2003).
Haberman, R. P., McCown, T. J. & Samulski, R. J. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 5, 1604–1611 (1998).
Scammell, T. E. et al. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J. Neurosci. 23, 5762–5770 (2003).
Ahmed, B. Y. et al. Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci. 5, 4 (2004).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Acknowledgements
The authors thank L. Enquist (Princeton) for helpful discussions and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks Eric Kremer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Marker genes
-
Genes whose cell type-specific mRNA and/or protein expression can be used to identify a cell type.
- Reporter genes
-
Genes whose expressed mRNA or protein is readily detectable and can be used to characterize another gene or gene product.
- Baltimore classification
-
A classification scheme (conceived by virologist David Baltimore) that characterizes a virus based on its nucleic acid composition, in particular how the virus ultimately synthesizes mRNA.
- Capsid
-
A proteinaceous shell that surrounds the viral genome.
- Vectors
-
Vehicles used to transfer nucleic acid information into the cell.
- Recombinase
-
An enzyme (for example, Cre or Flp) capable of recombining defined nucleic acid sequences, often used to activate or inactivate expression of a gene engineered to include sequences specific for that recombinase.
- Pseudotyping
-
The process of heterologously expressing proteins on a virus’ capsid/envelope (often with the intention of gaining advantageous viral spread/entry properties).
- Constructs
-
Designer DNA sequences.
- Cre-driver line
-
A mouse or rat strain (for example, knock-in and BAC transgenic) engineered to drive expression of Cre recombinase in a cell type-specific pattern (often using a marker gene’s promoter or enhancer elements).
Rights and permissions
About this article
Cite this article
Nectow, A.R., Nestler, E.J. Viral tools for neuroscience. Nat Rev Neurosci 21, 669–681 (2020). https://doi.org/10.1038/s41583-020-00382-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-020-00382-z
This article is cited by
-
A shedding analysis after AAV8 CNS injection revealed fragmented viral DNA without evidence of functional AAV particles in mice
Gene Therapy (2024)
-
A toolbox of astrocyte-specific, serotype-independent adeno-associated viral vectors using microRNA targeting sequences
Nature Communications (2023)
-
A custom-made AAV1 variant (AAV1-T593K) enables efficient transduction of Japanese quail neurons in vitro and in vivo
Communications Biology (2023)
-
Meeting report for the 2022 UC Irvine Center for neural circuit mapping conference: linking brain function to cell types and circuits
Molecular Psychiatry (2023)
-
Advanced neurobiological tools to interrogate metabolism
Nature Reviews Endocrinology (2023)