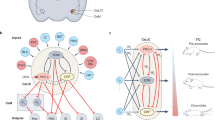
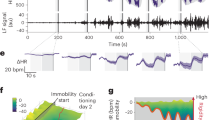
Abstract
Most animals live under constant threat from predators, and predation has been a major selective force in shaping animal behaviour. Nevertheless, defence responses against predatory threats need to be balanced against other adaptive behaviours such as foraging, mating and recovering from infection. This behavioural balance in ethologically relevant contexts requires adequate integration of internal and external signals in a complex interplay between the brain and the body. Despite this complexity, research has often considered defensive behaviour as entirely mediated by the brain processing threat-related information obtained via perception of the external environment. However, accumulating evidence suggests that the endocrine, immune, gastrointestinal and reproductive systems have important roles in modulating behavioural responses to threat. In this Review, we focus on how predatory threat defence responses are shaped by threat imminence and review the circuitry between subcortical brain regions involved in mediating defensive behaviours. Then, we discuss the intersection of peripheral systems involved in internal states related to infection, hunger and mating with the neurocircuits that underlie defence responses against predatory threat. Through this process, we aim to elucidate the interconnections between the brain and body as an integrated network that facilitates appropriate defensive responses to threat and to discuss the implications for future behavioural research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kozlowska, K., Walker, P., McLean, L. & Carrive, P. Fear and the defense cascade: clinical implications and management. Harv. Rev. Psychiatry 23, 263–287 (2015).
Bolles, R. C. Species-specific defense reactions and avoidance learning. Psychol. Rev. 77, 32–48 (1970).
Miller, N. E. Studies of fear as an acquirable drive: I. Fear as motivation and fear-reduction as reinforcement in the learning of new responses. J. Exp. Psychol. Gen. 38, 89–101 (1948).
Mowrer, O. H. Two-factor learning theory: summary and comment. Psychol. Rev. 58, 350–354 (1951).
Bolles, R. C. & Fanselow, M. S. A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci. 3, 291–301 (1980).
Fanselow, M. S. & Lester, L. S. in Evolution and Learning (eds Bolles, R. C. & Beecher, M. D.) 185–212 (Lawrence Erlbaum Associates, 1988).
Qi, S. et al. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl Acad. Sci. USA 115, 3186–3191 (2018).
Vieira, J. B., Schellhaas, S., Enström, E. & Olsson, A. Help or flight? Increased threat imminence promotes defensive helping in humans. Proc. Biol. Sci. 287, 20201473 (2020).
Mobbs, D., Headley, D. B., Ding, W. & Dayan, P. Space, time, and fear: survival computations along defensive circuits. Trends Cogn. Sci. 24, 228–241 (2020).
Kawai, N., Kono, R. & Sugimoto, S. Avoidance learning in the crayfish (Procambarus clarkii) depends on the predatory imminence of the unconditioned stimulus: a behavior systems approach to learning in invertebrates. Behav. Brain Res. 150, 229–237 (2004).
Fanselow, M. S. Negative valence systems: sustained threat and the predatory imminence continuum. Emerg. Top. Life Sci. 6, 467–477 (2022).
Cantor, C. Post-traumatic stress disorder: evolutionary perspectives. Aust. N. Z. J. Psychiatry 43, 1038–1048 (2009).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Alves de Lima, K. et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21, 1421–1429 (2020).
Larson, S. J. & Dunn, A. J. Behavioral effects of cytokines. Brain Behav. Immun. 15, 371–387 (2001).
Dantzer, R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 15, 7–24 (2001).
Alzarea, S. & Rahman, S. Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav. Brain Res. 366, 19–28 (2019).
Wieczorek, M., Swiergiel, A. H., Pournajafi-Nazarloo, H. & Dunn, A. J. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol. Behav. 85, 500–511 (2005).
Tillinger, A. & Mravec, B. Vagotomy affects lipopolysaccharide-induced changes of urocortin 2 gene expression in the brain and on the periphery. Neurochem. Res. 46, 159–164 (2021).
Bercik, P. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 23, 1132–1139 (2011).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Tanida, M. et al. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 389, 109–114 (2005).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Bitran, D., Kellogg, C. K. & Hilvers, R. J. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm. Behav. 27, 568–583 (1993).
Singh, D. K., Hari Dass, S. A., Abdulai-Saiku, S. & Vyas, A. Testosterone acts within the medial amygdala of rats to reduce innate fear to predator odor akin to the effects of Toxoplasma gondii infection. Front. Psychiatry 11, 630 (2020).
Frye, C. A. & Seliga, A. M. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 1, 371–381 (2001).
Pentkowski, N. S., Litvin, Y., Blanchard, D. C. & Blanchard, R. J. Effects of estrus cycle stage on defensive behavior in female Long–Evans hooded rats. Physiol. Behav. 194, 41–47 (2018).
Pham, T. A. & Lawley, T. D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 17, 67–74 (2014).
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
Cummings, D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Comeras, L. B., Herzog, H. & Tasan, R. O. Neuropeptides at the crossroad of fear and hunger: a special focus on neuropeptide Y. Ann. N. Y. Acad. Sci. 1455, 59–80 (2019).
Verma, D. et al. Hunger promotes fear extinction by activation of an amygdala microcircuit. Neuropsychopharmacology 41, 431–439 (2016).
Huang, C. C., Chou, D., Yeh, C. M. & Hsu, K. S. Acute food deprivation enhances fear extinction but inhibits long-term depression in the lateral amygdala via ghrelin signaling. Neuropharmacology 101, 36–45 (2016).
Jensen, M. et al. Anxiolytic-like effects of increased ghrelin receptor signaling in the amygdala. Int. J. Neuropsychopharmacol. 19, pyv123 (2016).
Tóth, K., László, K., Lukács, E. & Lénárd, L. Intraamygdaloid microinjection of acylated-ghrelin influences passive avoidance learning. Behav. Brain Res. 202, 308–311 (2009).
Flavell, S. W., Gogolla, N., Lovett-Barron, M. & Zelikowsky, M. The emergence and influence of internal states. Neuron 110, 2545–2570 (2022).
Marx, W., Moseley, G., Berk, M. & Jacka, F. Nutritional psychiatry: the present state of the evidence. Proc. Nutr. Soc. 76, 427–436 (2017).
Tang, F., Wang, G. & Lian, Y. Association between anxiety and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Psychoneuroendocrinology 77, 112–121 (2017).
Wang, J. et al. Influence of gut microbiota on resilience and its possible mechanisms. Int. J. Biol. Sci. 19, 2588–2598 (2023).
Michopoulos, V., Powers, A., Gillespie, C. F., Ressler, K. J. & Jovanovic, T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254–270 (2017).
Colombetti, G. & Zavala, E. Are emotional states based in the brain? A critique of affective brainocentrism from a physiological perspective. Biol. Philos. 34, 45 (2019).
Carr, J. A. I’ll take the low road: the evolutionary underpinnings of visually triggered fear. Front. Neurosci. 9, 414 (2015).
Blanchard, D. C., Hynd, A. L., Minke, K. A., Minemoto, T. & Blanchard, R. J. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neurosci. Biobehav. Rev. 25, 761–770 (2001).
Perusini, J. N. & Fanselow, M. S. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn. Mem. 22, 417–425 (2015).
Moscarello, J. M. & Penzo, M. A. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat. Neurosci. 25, 999–1008 (2022).
Roelofs, K. & Dayan, P. Freezing revisited: coordinated autonomic and central optimization of threat coping. Nat. Rev. Neurosci. 23, 568–580 (2022).
Mobbs, D. et al. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 29, 12236–12243 (2009).
Lerner, M. Comparative aspects of human and animal hypnosis. Am. J. Clin. Hypn. 5, 57–60 (1962).
Blanchard, D. C. & Blanchard, R. J. Ethoexperimental approaches to the biology of emotion. Annu. Rev. Psychol. 39, 43–68 (1988).
Lang, P. J. et al. (eds) Attention and Orienting: Sensory and Motivational Processes (Lawrence Erlbaum Associates, 1997).
Li, Z. et al. Corticostriatal control of defense behavior in mice induced by auditory looming cues. Nat. Commun. 12, 1040 (2021).
Wei, P. et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6, 6756 (2015).
Shang, C. et al. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat. Commun. 9, 1232 (2018).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018).
Zelikowsky, M. et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e19 (2018).
Blanchard, R. J. & Blanchard, D. C. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog. Neuropsychopharmacol. Biol. Psychiatry 13, S3–S14 (1989).
Bracha, H. S. Freeze, flight, fight, fright, faint: adaptationist perspectives on the acute stress response spectrum. CNS Spectr. 9, 679–685 (2004).
Bracha, H. S., Ralston, T. C., Matsukawa, J. M., Williams, A. E. & Bracha, A. S. Does “fight or flight” need updating? Psychosomatics 45, 448–449 (2004).
Humphreys, R. K. & Ruxton, G. D. A review of thanatosis (death feigning) as an anti-predator behaviour. Behav. Ecol. Sociobiol. 72, 22 (2018).
Carli, G. & Farabollini, F. (eds) Defence from Invertebrates to Mammals: Focus on Tonic Immobility Vol. 271 (Elsevier, 2022).
Crawford, M. & Masterson, F. A. Species-specific defense reactions and avoidance learning. An evaluative review. Pavlov. J. Biol. Sci. 17, 204–214 (1982).
Blanchard, D. C., Blanchard, R. J. & Griebel, G. Defensive responses to predator threat in the rat and mouse. Curr. Protoc. Neurosci. https://doi.org/10.1002/0471142301.ns0819s30 (2005).
Blanchard, D. C., Yang, M., Hebert, M. & Blanchard, R. J. in Encyclopedia of Stress 2nd edn (ed Fink, G. J.) pp. 722–726 (Academic, 2007).
Mobbs, D., Hagan, C. C., Dalgleish, T., Silston, B. & Prévost, C. The ecology of human fear: survival optimization and the nervous system. Front. Neurosci. 9, 55 (2015).
Martínez-García, F. & Lanuza, E. Evolution of vertebrate survival circuits. Curr. Opin. Behav. Sci. 24, 113–123 (2018).
Sewards, T. V. & Sewards, M. A. Innate visual object recognition in vertebrates: some proposed pathways and mechanisms. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 132, 861–891 (2002).
Vagnoni, E., Lourenco, S. F. & Longo, M. R. Threat modulates perception of looming visual stimuli. Curr. Biol. 22, R826–R827 (2012).
Blanchard, R. J. & Blanchard, D. C. Defensive reactions in the albino rat. Learn. Motiv. 2, 351–362 (1971).
Yilmaz, M. & Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013).
Schiff, W., Caviness, J. A. & Gibson, J. J. Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science 136, 982–983 (1962).
Ghazanfar, A. A., Neuhoff, J. G. & Logothetis, N. K. Auditory looming perception in rhesus monkeys. Proc. Natl Acad. Sci. USA 99, 15755–15757 (2002).
Maier, J. X., Neuhoff, J. G., Logothetis, N. K. & Ghazanfar, A. A. Multisensory integration of looming signals by rhesus monkeys. Neuron 43, 177–181 (2004).
Romei, V., Murray, M. M., Cappe, C. & Thut, G. Preperceptual and stimulus-selective enhancement of low-level human visual cortex excitability by sounds. Curr. Biol. 19, 1799–1805 (2009).
Baumgartner, R. et al. Asymmetries in behavioral and neural responses to spectral cues demonstrate the generality of auditory looming bias. Proc. Natl Acad. Sci. USA 114, 9743–9748 (2017).
Basso, M. A., Bickford, M. E. & Cang, J. Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron 109, 918–937 (2021).
De Franceschi, G., Vivattanasarn, T., Saleem, A. B. & Solomon, S. G. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154 (2016).
Wang, F., Li, E., De, L., Wu, Q. & Zhang, Y. OFF-transient alpha RGCs mediate looming triggered innate defensive response. Curr. Biol. 31, 2263–2273.e2263 (2021).
Cai, D., Luo, X., Shen, K. & Shen, Y. GABAergic retinal ganglion cells regulate innate defensive responses. Neuroreport 32, 643–649 (2021).
Perry, V. H. & Cowey, A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 12, 1125–1137 (1984).
Wurtz, R. H. & Goldberg, M. E. Superior colliculus cell responses related to eye movements in awake monkeys. Science 171, 82–84 (1971).
Stein, B. E. Development of the superior colliculus. Annu. Rev. Neurosci. 7, 95–125 (1984).
Koller, K., Rafal, R. D., Platt, A. & Mitchell, N. D. Orienting toward threat: contributions of a subcortical pathway transmitting retinal afferents to the amygdala via the superior colliculus and pulvinar. Neuropsychologia 128, 78–86 (2019).
Shang, C. et al. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015).
Zhou, Z. et al. A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron 103, 473–488.e6 (2019).
Terburg, D. et al. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell 175, 723–735.e16 (2018).
McFadyen, J., Dolan, R. J. & Garrido, M. I. The influence of subcortical shortcuts on disordered sensory and cognitive processing. Nat. Rev. Neurosci. 21, 264–276 (2020).
LeDoux, J. & Daw, N. D. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 19, 269–282 (2018).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016).
Lefler, Y., Campagner, D. & Branco, T. The role of the periaqueductal gray in escape behavior. Curr. Opin. Neurobiol. 60, 115–121 (2020).
Bandler, R. & Carrive, P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 439, 95–106 (1988).
Meller, S. T. & Dennis, B. J. Afferent projections to the periaqueductal gray in the rabbit. Neuroscience 19, 927–964 (1986).
Klop, E. M., Mouton, L. J. & Holstege, G. Periparabigeminal and adjoining mesencephalic tegmental field projections to the dorsolateral periaqueductal grey in cat — a possible role for oculomotor input in the defensive system. Eur. J. Neurosci. 23, 2145–2157 (2006).
Fanselow, M. S., Decola, J. P., De Oca, B. M. & Landeira-Fernandez, J. Ventral and dorsolateral regions of the midbrain periaqueductal gray (PAG) control different stages of defensive behavior: dorsolateral PAG lesions enhance the defensive freezing produced by massed and immediate shock. Aggress. Behav. 21, 63–77 (1995).
Mobbs, D. et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083 (2007).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Kennedy, A. et al. Stimulus-specific hypothalamic encoding of a persistent defensive state. Nature 586, 730–734 (2020).
Wang, L., Chen, I. Z. & Lin, D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358 (2015).
Wilent, W. B. et al. Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note. J. Neurosurg. 115, 295–300 (2011).
Wilent, W. B. et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. J. Neurosurg. 112, 1295–1298 (2010).
Wang, W. et al. Dorsal premammillary projection to periaqueductal gray controls escape vigor from innate and conditioned threats. eLife 10, e69178 (2021).
Kim, D. J., Lee, A. S., Yttredahl, A. A., Gómez-Rodríguez, R. & Anderson, B. J. Repeated threat (without direct harm) alters metabolic capacity in select regions that drive defensive behavior. Neuroscience 353, 106–118 (2017).
Cezario, A. F., Ribeiro-Barbosa, E. R., Baldo, M. V. & Canteras, N. S. Hypothalamic sites responding to predator threats — the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. Eur. J. Neurosci. 28, 1003–1015 (2008).
Wang, W. et al. Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats. Neuron 109, 1848–1860.e8 (2021).
Xie, Z. et al. Mechanically evoked defensive attack is controlled by GABAergic neurons in the anterior hypothalamic nucleus. Nat. Neurosci. 25, 72–85 (2022).
Rossier, D., La Franca, V., Salemi, T., Natale, S. & Gross, C. T. A neural circuit for competing approach and defense underlying prey capture. Proc. Natl Acad. Sci. USA 118, e2013411118 (2021).
Barbano, M. F. et al. VTA glutamatergic neurons mediate innate defensive behaviors. Neuron 107, 368–382.e8 (2020).
Chen, S. Y. et al. Control of behavioral arousal and defense by a glutamatergic midbrain-amygdala pathway in mice. Front. Neurosci. 16, 850193 (2022).
Daviu, N. et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 23, 398–410 (2020).
Tseng, Y. T. et al. The subthalamic corticotropin-releasing hormone neurons mediate adaptive REM-sleep responses to threat. Neuron 110, 1223–1239.e8 (2022).
Monassi, C. R., Leite-Panissi, C. R. & Menescal-de-Oliveira, L. Ventrolateral periaqueductal gray matter and the control of tonic immobility. Brain Res. Bull. 50, 201–208 (1999).
Donatti, A. F. & Leite-Panissi, C. R. GABAergic antagonist blocks the reduction of tonic immobility behavior induced by activation of 5-HT2 receptors in the basolateral nucleus of the amygdala in guinea pigs. Brain Res. Bull. 79, 358–364 (2009).
de Oliveira, L., Hoffmann, A. & Menescal-de-Oliveira, L. The lateral hypothalamus in the modulation of tonic immobility in guinea pigs. Neuroreport 8, 3489–3493 (1997).
Griessner, J. et al. Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Mol. Psychiatry 26, 534–544 (2021).
Sun, Y., Qian, L., Xu, L., Hunt, S. & Sah, P. Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala. Mol. Psychiatry https://doi.org/10.1038/s41380-020-00894-1 (2020).
Adhikari, A. et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179–185 (2015).
Xiao, Q. et al. A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Mol. Psychiatry 26, 4719–4741 (2021).
Avery, S. N., Clauss, J. A. & Blackford, J. U. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141 (2016).
Kim, S.-Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).
Andreatta, M. et al. Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63, 352–363 (2015).
Buff, C. et al. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci. 12, 1766–1774 (2017).
Deng, H., Xiao, X. & Wang, Z. Periaqueductal gray neuronal activities underlie different aspects of defensive behaviors. J. Neurosci. 36, 7580–7588 (2016).
Sukikara, M. H., Mota-Ortiz, S. R., Baldo, M. V., Felicio, L. F. & Canteras, N. S. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behav. Brain Res. 209, 226–233 (2010).
Damasio, A. & Carvalho, G. B. The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 14, 143–152 (2013).
Evans, D. A., Stempel, A. V., Vale, R. & Branco, T. Cognitive control of escape behaviour. Trends Cogn. Sci. 23, 334–348 (2019).
Quagliato, L. A. & Nardi, A. E. Cytokine alterations in panic disorder: a systematic review. J. Affect. Disord. 228, 91–96 (2018).
Banks, W. A., Kastin, A. J. & Broadwell, R. D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 2, 241–248 (1995).
Miller, A. H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav. Immun. 23, 149–158 (2009).
Engler, H. et al. Acute amygdaloid response to systemic inflammation. Brain Behav. Immun. 25, 1384–1392 (2011).
Hassanain, M., Bhatt, S., Zalcman, S. & Siegel, A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 1048, 1–11 (2005).
Bhatt, S., Bhatt, R., Zalcman, S. S. & Siegel, A. Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain Behav. Immun. 22, 224–233 (2008).
Steinberg, B. E. et al. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron. Med. 3, 7–17 (2016).
Salvador, A. F., de Lima, K. A. & Kipnis, J. Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol. 21, 526–541 (2021).
Wan, W., Wetmore, L., Sorensen, C. M., Greenberg, A. H. & Nance, D. M. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 34, 7–14 (1994).
Niijima, A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 61, 287–291 (1996).
Kurosawa, M., Uvnäs-Moberg, K., Miyasaka, K. & Lundeberg, T. Interleukin-1 increases activity of the gastric vagal afferent nerve partly via stimulation of type A CCK receptor in anesthetized rats. J. Auton. Nerv. Syst. 62, 72–78 (1997).
Ericsson, A., Kovács, K. J. & Sawchenko, P. E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 14, 897–913 (1994).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17 (2000).
Kawai, Y. Differential ascending projections from the male rat caudal nucleus of the tractus solitarius: an interface between local microcircuits and global macrocircuits. Front. Neuroanat. 12, 63 (2018).
Ghosal, S., Bundzikova-Osacka, J., Dolgas, C. M., Myers, B. & Herman, J. P. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology 45, 142–153 (2014).
Holt, M. K., Valderrama, N., Polanco, M. J. & Rinaman, L. Modulation of stress-related behaviour by hypothalamic engagement of preproglucagon neurons in the nucleus of the solitary tract. Preprint at bioRxiv https://doi.org/10.1101/2022.02.04.479117 (2022).
Harrison, N. A. et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414 (2009).
Clark, S. M. et al. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behav. Immun. 38, 192–201 (2014).
Fan, K. Q. et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879.e19 (2019).
Brebner, K., Hayley, S., Zacharko, R., Merali, Z. & Anisman, H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 22, 566–580 (2000).
Manley, K., Han, W., Zelin, G. & Lawrence, D. A. Crosstalk between the immune, endocrine, and nervous systems in immunotoxicology. Curr. Opin. Toxicol. 10, 37–45 (2018).
Sternberg, E. M., Chrousos, G. P., Wilder, R. L. & Gold, P. W. The stress response and the regulation of inflammatory disease. Ann. Intern. Med. 117, 854–866 (1992).
Swanson, L. W. & Sawchenko, P. E. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31, 410–417 (1980).
Bains, J. S., Wamsteeker Cusulin, J. I. & Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 16, 377–388 (2015).
Denver, R. J. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann. N. Y. Acad. Sci. 1163, 1–16 (2009).
Gentsch, C., Lichtsteiner, M. & Feer, H. Locomotor activity, defecation score and corticosterone levels during an openfield exposure: a comparison among individually and group-housed rats, and genetically selected rat lines. Physiol. Behav. 27, 183–186 (1981).
Myers, B., McKlveen, J. M. & Herman, J. P. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front. Neuroendocrinol. 35, 180–196 (2014).
Kalin, N. H., Shelton, S. E., Rickman, M. & Davidson, R. J. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav. Neurosci. 112, 251–254 (1998).
Buss, K. A., Davidson, R. J., Kalin, N. H. & Goldsmith, H. H. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Dev. Psychol. 40, 583–594 (2004).
Walf, A. A. & Frye, C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328 (2007).
Lyte, M., Li, W., Opitz, N., Gaykema, R. P. & Goehler, L. E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 89, 350–357 (2006).
Kittana, H. et al. Commensal Escherichia coli strains can promote intestinal inflammation via differential interleukin-6 production. Front. Immunol. 9, 2318 (2018).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
Needham, B. D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022).
Burnett, C. J. et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016).
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Baver, S. B. et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 34, 5486–5496 (2014).
Deem, J. D., Faber, C. L. & Morton, G. J. AgRP neurons: regulators of feeding, energy expenditure, and behavior. FEBS J. 289, 2362–2381 (2022).
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Aklan, I. et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 31, 313–326.e315 (2020).
Fritz, E. M., Singewald, N. & De Bundel, D. The good, the bad and the unknown aspects of ghrelin in stress coping and stress-related psychiatric disorders. Front. Synaptic Neurosci. 12, 594484 (2020).
Kharitonenkov, A. & DiMarchi, R. FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends Endocrinol. Metab. 26, 608–617 (2015).
Fisher, F. M. & Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 78, 223–241 (2016).
Sa-Nguanmoo, P., Chattipakorn, N. & Chattipakorn, S. C. Potential roles of fibroblast growth factor 21 in the brain. Metab. Brain Dis. 31, 239–248 (2016).
Usui, N. et al. Roles of fibroblast growth factor 21 in the control of depression-like behaviours after social defeat stress in male rodents. J. Neuroendocrinol. 33, e13026 (2021).
Wang, Y. et al. Exposure of male mice to perfluorooctanoic acid induces anxiety-like behaviors by increasing corticotropin-releasing factor in the basolateral amygdala complex. Chemosphere 287, 132170 (2022).
Frederich, R. C. et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J. Clin. Invest. 96, 1658–1663 (1995).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Finger, B. C., Dinan, T. G. & Cryan, J. F. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology 210, 559–568 (2010).
Liu, J., Perez, S. M., Zhang, W., Lodge, D. J. & Lu, X. Y. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol. Psychiatry 16, 1024–1038 (2011).
Wang, W. et al. Leptin: a potential anxiolytic by facilitation of fear extinction. CNS Neurosci. Ther. 21, 425–434 (2015).
Harvey, J. Leptin: a diverse regulator of neuronal function. J. Neurochem. 100, 307–313 (2007).
Appleton, J. The gut-brain axis: influence of microbiota on mood and mental health. Integr. Med. 17, 28–32 (2018).
Leeuwendaal, N. K., Cryan, J. F. & Schellekens, H. Gut peptides and the microbiome: focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 28, 243–252 (2021).
Asakawa, A. et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74, 143–147 (2001).
Spencer, S. J. et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry 72, 457–465 (2012).
Heiman, M. L. et al. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138, 3859–3863 (1997).
Bonnavion, P., Jackson, A. C., Carter, M. E. & de Lecea, L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun. 6, 6266 (2015).
Liang, Q. et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075 (2014).
Hsuchou, H., Pan, W. & Kastin, A. J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386 (2007).
Kaprara, A. & Huhtaniemi, I. T. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism 86, 3–17 (2018).
Roselli, C. F. Brain aromatase: roles in reproduction and neuroprotection. J. Steroid Biochem. Mol. Biol. 106, 143–150 (2007).
Celotti, F., Negri-Cesi, P. & Poletti, A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res. Bull. 44, 365–375 (1997).
Kauffman, A. S. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front. Neurosci. 16, 953252 (2022).
Fink, G. Oestrogen and progesterone interactions in the control of gonadotrophin and prolactin secretion. J. Steroid Biochem. 30, 169–178 (1988).
Harding, C. F. Social modulation of circulating hormone levels in the male1. Am. Zool. 21, 223–231 (2015).
Grebe, N. M., Sarafin, R. E., Strenth, C. R. & Zilioli, S. Pair-bonding, fatherhood, and the role of testosterone: a meta-analytic review. Neurosci. Biobehav. Rev. 98, 221–233 (2019).
Ventura-Aquino, E., Fernández-Guasti, A. & Paredes, R. G. Hormones and the Coolidge effect. Mol. Cell. Endocrinol. 467, 42–48 (2018).
He, F., Yu, P. & Wu, R. Relationship between sexual satiety and motivation, brain androgen receptors and testosterone in male mandarin voles. Behav. Brain Res. 250, 257–263 (2013).
Aikey, J. L., Nyby, J. G., Anmuth, D. M. & James, P. J. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 42, 448–460 (2002).
Tong, W. H., Abdulai-Saiku, S. & Vyas, A. Testosterone reduces fear and causes drastic hypomethylation of arginine vasopressin promoter in medial extended amygdala of male mice. Front. Behav. Neurosci. 13, 33 (2019).
Auger, C. J., Coss, D., Auger, A. P. & Forbes-Lorman, R. M. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc. Natl Acad. Sci. USA 108, 4242–4247 (2011).
Viau, V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J. Neuroendocrinol. 14, 506–513 (2002).
Magnhagen, C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186 (1991).
Handy, A. B., Greenfield, S. F., Yonkers, K. A. & Payne, L. A. Psychiatric symptoms across the menstrual cycle in adult women: a comprehensive review. Harv. Rev. Psychiatry 30, 100–117 (2022).
Nillni, Y. I., Rasmusson, A. M., Paul, E. L. & Pineles, S. L. The impact of the menstrual cycle and underlying hormones in anxiety and PTSD: what do we know and where do we go from here? Curr. Psychiatry Rep. 23, 8 (2021).
Green, S. A. & Graham, B. M. Symptom fluctuation over the menstrual cycle in anxiety disorders, PTSD, and OCD: a systematic review. Arch. Women’s Ment. Health 25, 71–85 (2022).
Dallman, M. F. et al. in Hormones, Brain and Behavior (eds Pfaff, D. W. et al.) 571–631 (Academic, 2002).
Nesse, R. M. Evolutionary psychiatry: foundations, progress and challenges. World Psychiatry 22, 177–202 (2023).
Smith, S. M. & Vale, W. W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 8, 383–395 (2006).
Silva, B. A., Gross, C. T. & Graff, J. The neural circuits of innate fear: detection, integration, action, and memorization. Learn. Mem. 23, 544–555 (2016).
Branco, T. & Redgrave, P. The neural basis of escape behavior in vertebrates. Annu. Rev. Neurosci. 43, 417–439 (2020).
Tang, F. et al. mRNA-seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Vazquez-Guardado, A., Yang, Y., Bandodkar, A. J. & Rogers, J. A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 23, 1522–1536 (2020).
Willmore, L., Cameron, C., Yang, J., Witten, I. B. & Falkner, A. L. Behavioural and dopaminergic signatures of resilience. Nature 611, 124–132 (2022).
Campagner, D. et al. A cortico-collicular circuit for orienting to shelter during escape. Nature 613, 111–119 (2023).
Signoret-Genest, J. et al. Integrated cardio-behavioral responses to threat define defensive states. Nat. Neurosci. 26, 447–457 (2023).
Wiltschko, A. B. et al. Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 (2015).
Wiltschko, A. B. et al. Revealing the structure of pharmacobehavioral space through motion sequencing. Nat. Neurosci. 23, 1433–1443 (2020).
Anderson, D. J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
Tseng, Y. T. et al. Systematic evaluation of a predator stress model of depression in mice using a hierarchical 3D-motion learning framework. Transl. Psychiatry 13, 178 (2023).
Urai, A. E., Doiron, B., Leifer, A. M. & Churchland, A. K. Large-scale neural recordings call for new insights to link brain and behavior. Nat. Neurosci. 25, 11–19 (2022).
Acknowledgements
The authors thank all scientists whose studies were reviewed in this paper and apologize to those whose work was not cited owing to space limitations. The authors thank B. H. Zhao for assistance with illustrations. This work was supported by grants from the National Natural Science Foundation of China (32230042 and 31930047 to L.W., 32200826 to Y.-T.T. and 32222036 to P.W.), the Shenzhen Science and Technology Program (KCXFZ20211020163549011 to B.S. and JCYJ20220530154412028 to Y.-T.T.) and the Financial Support for Outstanding Talents Training Fund in Shenzhen (L.W.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Moriel Zelikowsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Adaptive behaviour
-
Behaviour is adaptive in an evolutionary sense if it increases genetic fitness, usually by promoting the survival and reproduction of an organism or that of close kin, which can coincide with negative emotional states such as anxiety or fear.
- Auditory looming assay
-
Behavioural test in which an animal is exposed to sudden increasing sounds induced by broadband white noise, triggering circa-strike phase defensive behaviours.
- Conditioned fear
-
A learned defensive response elicited by a previously neutral stimulus (conditioned stimulus) that has been paired with an aversive event (unconditioned stimulus).
- Defence vigour
-
Physical strength of the execution of defensive behaviours. For example, an increase in speed during escape and increased durations of avoidance or persistent freezing can be considered increased defence vigour.
- Dysbiotic microbiota
-
An imbalance or disruption in bacterial composition, metabolic activities or distribution within the intestinal tract, often leading to negative health consequences such as inflammation and disease.
- Elevated plus maze
-
Behavioural test in which an animal explores elevated open and enclosed arms and in which reduced entries into open arms indicate enhanced defensive behaviour in the pre-encounter phase and an anxiety-like state.
- Maladaptive behaviour
-
Behaviour patterns that are adaptive in some contexts can become maladaptive in an evolutionary sense when they are displayed in the wrong context or with excessive vigour and, thereby, prevent the execution of adequate adaptive behaviours, ultimately reducing the survival chances and reproductive success of the organism.
- Open field test
-
Behavioural test in which an animal explores an unstructured open arena and the increased avoidance of central areas indicates enhanced defensive behaviours in the pre-encounter phase (often referred to as anxiety-related behaviour).
- Optogenetics
-
An approach involving the expression of light-sensitive ion channels or pumps in specific cells, allowing cellular or organ activity to be manipulated by light with high spatial and temporal precision.
- Phase shifts in the threat imminence continuum
-
Phase shifts in the threat imminence continuum refer to the phenomenon when behaviour patterns typical for one phase of the continuum are activated earlier (at lower imminence) or later (at higher imminence) than typically observed. They may be adaptive in some contexts or maladaptive in others.
- Visual looming assay
-
Behavioural test in which an animal is exposed to rapidly expanding dark overhead spots that serve as visual cues simulating approaching threats, triggering circa-strike phase defensive behaviours.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tseng, YT., Schaefke, B., Wei, P. et al. Defensive responses: behaviour, the brain and the body. Nat. Rev. Neurosci. 24, 655–671 (2023). https://doi.org/10.1038/s41583-023-00736-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00736-3