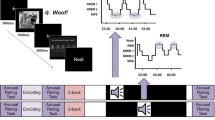
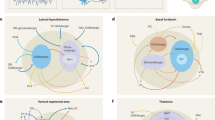
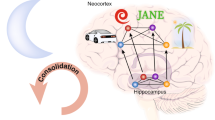
Abstract
Expressions such as ‘sleep on it’ refer to the resolution of distressing experiences across a night of sound sleep. Sleep is an active state during which the brain reorganizes the synaptic connections that form memories. This Perspective proposes a model of how sleep modifies emotional memory traces. Sleep-dependent reorganization occurs through neurophysiological events in neurochemical contexts that determine the fates of synapses to grow, to survive or to be pruned. We discuss how low levels of acetylcholine during non-rapid eye movement sleep and low levels of noradrenaline during rapid eye movement sleep provide a unique window of opportunity for plasticity in neuronal representations of emotional memories that resolves the associated distress. We integrate sleep-facilitated adaptation over three levels: experience and behaviour, neuronal circuits, and synaptic events. The model generates testable hypotheses for how failed sleep-dependent adaptation to emotional distress is key to mental disorders, notably disorders of anxiety, depression and post-traumatic stress with the common aetiology of insomnia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hamann, S. Cognitive and neural mechanisms of emotional memory. Trends Cogn. Sci. 5, 394–400 (2001).
LaBar, K. S. & Cabeza, R. Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 7, 54–64 (2006).
Crowley, R., Bendor, D. & Javadi, A.-H. A review of neurobiological factors underlying the selective enhancement of memory at encoding, consolidation, and retrieval. Prog. Neurobiol. 179, 101615 (2019).
Ashton, J. E., Harrington, M. O., Guttesen, A. Á. V., Smith, A. K. & Cairney, S. A. Sleep preserves physiological arousal in emotional memory. Sci. Rep. 9, 5966 (2019).
Bolinger, E. et al. Sleep’s benefits to emotional processing emerge in the long term. Cortex 120, 457–470 (2019).
Lipinska, G. & Thomas, K. G. F. The interaction of REM fragmentation and night-time arousal modulates sleep-dependent emotional memory consolidation. Front. Psychol. 10, 1766 (2019).
Conte, F., Cerasuolo, M., Giganti, F. & Ficca, G. Sleep enhances strategic thinking at the expense of basic procedural skills consolidation. J. Sleep Res. 29, e13034 (2020).
Tucker, M. A., Humiston, G. B., Summer, T. & Wamsley, E. Comparing the effects of sleep and rest on memory consolidation. Nat. Sci. Sleep 12, 79–91 (2020).
Short, M. A., Booth, S. A., Omar, O., Ostlundh, L. & Arora, T. The relationship between sleep duration and mood in adolescents: a systematic review and meta-analysis. Sleep Med. Rev. 52, 101311 (2020).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014).
Niethard, N. & Born, J. Back to baseline: sleep recalibrates synapses. Nat. Neurosci. 22, 149–151 (2019).
Genzel, L., Kroes, M. C. W., Dresler, M. & Battaglia, F. P. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 37, 10–19 (2014).
Osorio-Forero, A., Cherrad, N., Banterle, L., Fernandez, L. M. J. & Lüthi, A. When the locus coeruleus speaks up in sleep: recent insights, emerging perspectives. Int. J. Mol. Sci. 23, 5028 (2022).
Kjaerby, C. et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 25, 1059–1070 (2022). This paper shows that long LC silences during sleep promote memory, sleep spindles and REM sleep, whereas shorter LC silences and more frequent increases in NA promote microarousals and decrease memory performance, thereby reinforcing the concept that NA absences are important to sleep-dependent memory consolidation.
Swift, K. M. et al. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol. 28, 3599–3609.e4 (2018). This paper shows how aberrant activity of the LC during normally silent times (that is, REM sleep and the transition to REM sleep period) disrupts spindle generation and weakens delta and theta oscillation power, interfering with proper learning.
Osorio-Forero, A. et al. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 31, 5009–5023.e7 (2021).
Wassing, R. et al. Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol. 29, 2351–2358.e4 (2019). This paper shows that overnight amygdala adaptation is proportional to the duration of sound REM sleep and preceding NREM sleep spindles, but that adaptation fails proportionally to the number of awakenings and arousals in REM sleep.
Poe, G. R. Sleep is for forgetting. J. Neurosci. 37, 464–473 (2017).
Guskjolen, A. & Cembrowski, M. S. Engram neurons: encoding, consolidation, retrieval, and forgetting of memory. Mol. Psychiatry 28, 3207–3219 (2023).
Roy, D. S. et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 13, 1799 (2022).
Ghosh, V. E. & Gilboa, A. What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia 53, 104–114 (2014).
van Kesteren, M. T. R., Rijpkema, M., Ruiter, D. J. & Fernández, G. Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. J. Neurosci. 30, 15888–15894 (2010).
Takashima, A. et al. Shift from hippocampal to neocortical centered retrieval network with consolidation. J. Neurosci. 29, 10087–10093 (2009).
Nieuwenhuis, I. L. C. & Takashima, A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res. 218, 325–334 (2011).
Lubenov, E. V. & Siapas, A. G. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron 58, 118–131 (2008).
Kim, J. et al. Amygdala depotentiation and fear extinction. Proc. Natl Acad. Sci. USA 104, 20955–20960 (2007).
Frey, U. & Morris, R. G. Synaptic tagging and long-term potentiation. Nature 385, 533–536 (1997).
Viola, H., Ballarini, F., Martínez, M. C. & Moncada, D. The tagging and capture hypothesis from synapse to memory. Prog. Mol. Biol. Transl. Sci. 122, 391–423 (2014).
Payne, J. D. & Kensinger, E. A. Stress, sleep, and the selective consolidation of emotional memories. Curr. Opin. Behav. Sci. 19, 36–43 (2018).
Cunningham, T. J. et al. Higher post-encoding cortisol benefits the selective consolidation of emotional aspects of memory. Neurobiol. Learn. Mem. 180, 107411 (2021).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Hua, S.-S. et al. NMDA receptor-dependent synaptic potentiation via APPL1 signaling is required for the accessibility of a prefrontal neuronal assembly in retrieving fear extinction. Biol. Psychiatry 94, 262–277 (2023).
Marek, R., Sun, Y. & Sah, P. Neural circuits for a top-down control of fear and extinction. Psychopharmacology 236, 313–320 (2019).
van Kesteren, M. T. R. et al. Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: from congruent to incongruent. Neuropsychologia 51, 2352–2359 (2013).
Sommer, T., Hennies, N., Lewis, P. A. & Alink, A. The assimilation of novel information into schemata and its efficient consolidation. J. Neurosci. 42, 5916–5929 (2022).
Cowan, E. et al. Sleep spindles promote the restructuring of memory representations in ventromedial prefrontal cortex through enhanced hippocampal-cortical functional connectivity. J. Neurosci. 40, 1909–1919 (2020).
Seibt, J. & Frank, M. G. Primed to sleep: the dynamics of synaptic plasticity across brain states. Front. Syst. Neurosci. 13, 2 (2019).
Cowan, E. T., Schapiro, A. C., Dunsmoor, J. E. & Murty, V. P. Memory consolidation as an adaptive process. Psychon. Bull. Rev. 28, 1796–1810 (2021).
Vanderheyden, W. M., Poe, G. R. & Liberzon, I. Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. Exp. Brain Res. 232, 1575–1584 (2014).
Makino, S., Hashimoto, K. & Gold, P. W. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol. Biochem. Behav. 73, 147–158 (2002).
Kaouane, N. et al. Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335, 1510–1513 (2012).
Atucha, E. et al. Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proc. Natl Acad. Sci. USA 114, 9176–9181 (2017).
Krenz, V., Sommer, T., Alink, A., Roozendaal, B. & Schwabe, L. Noradrenergic arousal after encoding reverses the course of systems consolidation in humans. Nat. Commun. 12, 6054 (2021).
Al Abed, A. S. et al. Preventing and treating PTSD-like memory by trauma contextualization. Nat. Commun. 11, 4220 (2020).
Desmedt, A., Marighetto, A. & Piazza, P.-V. Abnormal fear memory as a model for posttraumatic stress disorder. Biol. Psychiatry 78, 290–297 (2015).
Lissek, S. et al. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 75, 909–915 (2014).
Foote, S. L., Aston-Jones, G. & Bloom, F. E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl Acad. Sci. USA 77, 3033–3037 (1980).
Grant, S. J., Aston-Jones, G. & Redmond, D. E. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res. Bull. 21, 401–410 (1988).
Sapolsky, R. M., Romero, L. M. & Munck, A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (2000).
Waterhouse, B. D. & Navarra, R. L. The locus coeruleus-norepinephrine system and sensory signal processing: a historical review and current perspectives. Brain Res. 1709, 1–15 (2019).
Axmacher, N., Mormann, F., Fernández, G., Elger, C. E. & Fell, J. Memory formation by neuronal synchronization. Brain Res. Rev. 52, 170–182 (2006).
Jutras, M. J. & Buffalo, E. A. Synchronous neural activity and memory formation. Curr. Opin. Neurobiol. 20, 150–155 (2010).
Fernández-Ruiz, A. et al. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron 93, 1213–1226.e5 (2017).
Costa, M. et al. Aversive memory formation in humans involves an amygdala-hippocampus phase code. Nat. Commun. 13, 6403 (2022).
Andrade-Talavera, Y., Fisahn, A. & Rodríguez-Moreno, A. Timing to be precise? An overview of spike timing-dependent plasticity, brain rhythmicity, and glial cells interplay within neuronal circuits. Mol. Psychiatry 28, 2177–2188 (2023).
Teyler, T. J. & Rudy, J. W. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus 17, 1158–1169 (2007).
Schlichting, M. L., Zeithamova, D. & Preston, A. R. CA1 subfield contributions to memory integration and inference. Hippocampus 24, 1248–1260 (2014).
Villano, W. J., Otto, A. R., Ezie, C. E. C., Gillis, R. & Heller, A. S. Temporal dynamics of real-world emotion are more strongly linked to prediction error than outcome. J. Exp. Psychol. Gen. 149, 1755–1766 (2020).
Simpkiss, J. L. & Devine, D. P. Responses of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neuro Endocrinol. Lett. 24, 97–103 (2003).
Wassing, R. et al. Haunted by the past: old emotions remain salient in insomnia disorder. Brain 142, 1783–1796 (2019).
Botvinick, M. M., Cohen, J. D. & Carter, C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8, 539–546 (2004).
Vinogradova, O. S. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11, 578–598 (2001).
Borisyuk, R., Denham, M., Hoppensteadt, F., Kazanovich, Y. & Vinogradova, O. An oscillatory neural network model of sparse distributed memory and novelty detection. BioSystems 58, 265–272 (2000).
Albasser, M. M., Poirier, G. L. & Aggleton, J. P. Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-Fos imaging. Eur. J. Neurosci. 31, 134–147 (2010).
Kinnavane, L., Amin, E., Olarte-Sánchez, C. M. & Aggleton, J. P. Detecting and discriminating novel objects: the impact of perirhinal cortex disconnection on hippocampal activity patterns. Hippocampus 26, 1393–1413 (2016).
Sara, S. J., Vankov, A. & Hervé, A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull. 35, 457–465 (1994).
Straube, T., Korz, V., Balschun, D. & Frey, J. U. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J. Physiol. 552, 953–960 (2003).
Rajkumar, R., Kumar, J. R. & Dawe, G. S. Priming locus coeruleus noradrenergic modulation of medial perforant path-dentate gyrus synaptic plasticity. Neurobiol. Learn. Mem. 138, 215–225 (2017).
Otmakhova, N. A. & Lisman, J. E. Dopamine, serotonin, and noradrenaline strongly inhibit the direct perforant path-CA1 synaptic input, but have little effect on the Schaffer collateral input. Ann. N. Y. Acad. Sci. 911, 462–464 (2000).
Xiao, Z. et al. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J. Biol. Chem. 284, 10980–10991 (2009).
Reyes, B. A. S., Carvalho, A. F., Vakharia, K. & Van Bockstaele, E. J. Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Exp. Neurol. 230, 96–105 (2011).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Lamotte, G., Shouman, K. & Benarroch, E. E. Stress and central autonomic network. Auton. Neurosci. 235, 102870 (2021).
Mather, M., Clewett, D., Sakaki, M. & Harley, C. W. Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 39, e200 (2016).
Dahl, M. J., Mather, M. & Werkle-Bergner, M. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci. 26, 38–52 (2022).
O’Dell, T. J., Connor, S. A., Guglietta, R. & Nguyen, P. V. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 22, 461–471 (2015).
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Castillo, P. E. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb. Perspect. Biol. 4, a005728 (2012).
Herring, B. E. & Nicoll, R. A. Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol. 78, 351–365 (2016).
Kessels, H. W. & Malinow, R. Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350 (2009).
Diering, G. H. & Huganir, R. L. The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018).
Wenthold, R. J., Petralia, R. S., Blahos J, I. I. & Niedzielski, A. S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 (1996).
Makino, H. & Malinow, R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron 72, 1001–1011 (2011).
Watt, A. J., Sjöström, P. J., Häusser, M., Nelson, S. B. & Turrigiano, G. G. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat. Neurosci. 7, 518–524 (2004).
Bellone, C. & Nicoll, R. A. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785 (2007).
Holehonnur, R. et al. Increasing the GluN2A/GluN2B ratio in neurons of the mouse basal and lateral amygdala inhibits the modification of an existing fear memory trace. J. Neurosci. 36, 9490–9504 (2016). This paper shows that memories are stabilized and, therefore, become more resistant to modification through an NMDA receptor subunit switch at synapses.
Murphy, J. A. et al. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J. Neurosci. 34, 869–879 (2014).
Renner, M. C. et al. Synaptic plasticity through activation of GluA3-containing AMPA-receptors. eLife 6, e25462 (2017).
Thomas, M. J., Moody, T. D., Makhinson, M. & O’Dell, T. J. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17, 475–482 (1996).
Katsuki, H., Izumi, Y. & Zorumski, C. F. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J. Neurophysiol. 77, 3013–3020 (1997).
Hu, H. et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131, 160–173 (2007).
Hruska, M., Cain, R. E. & Dalva, M. B. Nanoscale rules governing the organization of glutamate receptors in spine synapses are subunit specific. Nat. Commun. 13, 920 (2022).
McReynolds, J. R., Anderson, K. M., Donowho, K. M. & McIntyre, C. K. Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiol. Learn. Mem. 115, 49–57 (2014).
Waltereit, R. et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J. Neurosci. 21, 5484–5493 (2001).
Chowdhury, S. et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52, 445–459 (2006).
Rial Verde, E. M., Lee-Osbourne, J., Worley, P. F., Malinow, R. & Cline, H. T. Increased expression of the immediate-early gene Arc/Arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52, 461–474 (2006).
Okuno, H. et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149, 886–898 (2012).
Okuno, H., Minatohara, K. & Bito, H. Inverse synaptic tagging: an inactive synapse-specific mechanism to capture activity-induced Arc/Arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin. Cell Dev. Biol. 77, 43–50 (2018).
El-Boustani, S. et al. Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science 360, 1349–1354 (2018).
Newpher, T. M., Harris, S., Pringle, J., Hamilton, C. & Soderling, S. Regulation of spine structural plasticity by Arc/Arg3.1. Semin. Cell Dev. Biol. 77, 25–32 (2018).
Holtmaat, A. J. G. D. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
Yang, G., Pan, F. & Gan, W.-B. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009).
Radley, J. J. et al. Associative Pavlovian conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the lateral amygdala. Eur. J. Neurosci. 24, 876–884 (2006).
Hill, T. C. & Zito, K. LTP-induced long-term stabilization of individual nascent dendritic spines. J. Neurosci. 33, 678–686 (2013).
Harley, C. W. & Milway, J. S. Glutamate ejection in the locus coeruleus enhances the perforant path-evoked population spike in the dentate gyrus. Exp. Brain Res. 63, 143–150 (1986).
Quinlan, M. A. L. et al. Locus coeruleus optogenetic light activation induces long-term potentiation of perforant path population spike amplitude in rat dentate gyrus. Front. Syst. Neurosci. 12, 67 (2018).
Lesuis, S. L., Timmermans, W., Lucassen, P. J., Hoogenraad, C. C. & Krugers, H. J. Glucocorticoid and β-adrenergic regulation of hippocampal dendritic spines. J. Neuroendocrinol. 32, e12811 (2020).
Genoux, D. et al. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418, 970–975 (2002).
Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014).
Moreno, A. Molecular mechanisms of forgetting. Eur. J. Neurosci. 54, 6912–6932 (2021).
Nabavi, S. et al. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc. Natl Acad. Sci. USA 110, 4027–4032 (2013).
Holman, D., Feligioni, M. & Henley, J. M. Differential redistribution of native AMPA receptor complexes following LTD induction in acute hippocampal slices. Neuropharmacology 52, 92–99 (2007).
Granger, A. J. & Nicoll, R. A. LTD expression is independent of glutamate receptor subtype. Front. Synaptic Neurosci. 6, 15 (2014).
Sanderson, T. M. Molecular mechanisms involved in depotentiation and their relevance to schizophrenia. Chonnam Med. J. 48, 1–6 (2012).
Man, H.-Y., Sekine-Aizawa, Y. & Huganir, R. L. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl Acad. Sci. USA 104, 3579–3584 (2007).
Aston-Jones, G. & Bloom, F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886 (1981).
Nitz, D. & Siegel, J. M. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience 78, 795–801 (1997).
Maquet, P. et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383, 163–166 (1996).
Braun, A. R. et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 120, 1173–1197 (1997).
Nofzinger, E. A. et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 125, 1105–1115 (2002).
Pace-Schott, E. F. & Hobson, J. A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3, 591–605 (2002).
Hasselmo, M. E. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn. Sci. 3, 351–359 (1999).
Clemens, Z. et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur. J. Neurosci. 33, 511–520 (2011).
Purcell, S. M. et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun. 8, 15930 (2017).
Fernandez, L. M. J. & Lüthi, A. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868 (2020).
Kim, J., Gulati, T. & Ganguly, K. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell 179, 514–526.e13 (2019).
Cox, R., Mylonas, D. S., Manoach, D. S. & Stickgold, R. Large-scale structure and individual fingerprints of locally coupled sleep oscillations. Sleep 41, zsy175 (2018).
Dehnavi, F., Koo-Poeggel, P. C., Ghorbani, M. & Marshall, L. Spontaneous slow oscillation-slow spindle features predict induced overnight memory retention. Sleep 44, zsab127 (2021).
McCormick, D. A. & Prince, D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J. Neurophysiol. 59, 978–996 (1988).
Lee, K. H. & McCormick, D. A. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron 17, 309–321 (1996).
Kjaerby, C. et al. Reply to: “Do all norepinephrine surges disrupt sleep?”. Nat. Neurosci. 26, 957–958 (2023).
Eschenko, O., Magri, C., Panzeri, S. & Sara, S. J. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 22, 426–435 (2012).
Brodt, S., Inostroza, M., Niethard, N. & Born, J. Sleep — a brain-state serving systems memory consolidation. Neuron 111, 1050–1075 (2023).
Hutchison, I. C. & Rathore, S. The role of REM sleep theta activity in emotional memory. Front. Psychol. 6, 1439 (2015).
Karashima, A. et al. Synchronization between hippocampal theta waves and PGO waves during REM sleep. Psychiatry Clin. Neurosci. 55, 189–190 (2001).
Karashima, A., Nakao, M., Katayama, N. & Honda, K. Instantaneous acceleration and amplification of hippocampal theta wave coincident with phasic pontine activities during REM sleep. Brain Res. 1051, 50–56 (2005).
Karashima, A., Katayama, N. & Nakao, M. Enhancement of synchronization between hippocampal and amygdala theta waves associated with pontine wave density. J. Neurophysiol. 103, 2318–2325 (2010).
Lesting, J. et al. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PloS ONE 6, e21714 (2011).
Lesting, J. et al. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PloS ONE 8, e77707 (2013). This paper reports that proper extinction memory recall results from theta oscillation coherence between the CA1 and amygdala that is phase locked to that of the mPFC. Rapid extinction learning occurred when the hippocampus and amygdala were anti-phase coupled.
Totty, M. S., Chesney, L. A., Geist, P. A. & Datta, S. Sleep-dependent oscillatory synchronization: a role in fear memory consolidation. Front. Neural Circuits 11, 49 (2017).
Datta, S., Saha, S., Prutzman, S. L., Mullins, O. J. & Mavanji, V. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J. Neurosci. Res. 80, 727–737 (2005).
Aime, M. et al. Paradoxical somatodendritic decoupling supports cortical plasticity during REM sleep. Science 376, 724–730 (2022). This series of studies demonstrates a REM sleep-specific amplification of distal dendrite activity in the prefrontal cortex at the same time that the perisomatic area of the same cells is silent — a process involving changed interneuronal activity levels which, when perturbed, prevent normal consolidation of emotional memories.
Brécier, A., Borel, M., Urbain, N. & Gentet, L. J. Vigilance and behavioral state-dependent modulation of cortical neuronal activity throughout the sleep/wake cycle. J. Neurosci. 42, 4852–4866 (2022).
Niethard, N., Ngo, H.-V. V., Ehrlich, I. & Born, J. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc. Natl Acad. Sci. USA 115, E9220–E9229 (2018).
Niethard, N., Brodt, S. & Born, J. Cell-type-specific dynamics of calcium activity in cortical circuits over the course of slow-wave sleep and rapid eye movement sleep. J. Neurosci. 41, 4212–4222 (2021).
Katona, L. et al. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82, 872–886 (2014).
Kjaerby, C. et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 25, 1059–1070 (2022).
Havekes, R. & Aton, S. J. Impacts of sleep loss versus waking experience on brain plasticity: parallel or orthogonal? Trends Neurosci. 43, 385–393 (2020).
Maret, S., Faraguna, U., Nelson, A. B., Cirelli, C. & Tononi, G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 14, 1418–1420 (2011).
de Vivo, L. et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017).
Vyazovskiy, V. V., Cirelli, C., Pfister-Genskow, M., Faraguna, U. & Tononi, G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 11, 200–208 (2008).
Diering, G. H. et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017).
Diering, G. H. Remembering and forgetting in sleep: selective synaptic plasticity during sleep driven by scaling factors Homer1a and Arc. Neurobiol. Stress 22, 100512 (2023).
Miyamoto, D., Marshall, W., Tononi, G. & Cirelli, C. Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex. Nat. Commun. 12, 2881 (2021). This paper reveals that those synapses that are potentiated the most by learning are also least inclined to be weakened during sleep.
Yang, G. et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014). This paper shows that cortical neurons that are activated during a learning task are reactivated during REM sleep, and that this reactivation is crucial for maintaining the newly formed spines at these neurons.
Seibt, J. et al. Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat. Commun. 8, 684 (2017).
Feld, G. B., Lange, T., Gais, S. & Born, J. Sleep-dependent declarative memory consolidation — unaffected after blocking NMDA or AMPA receptors but enhanced by NMDA coagonist D-cycloserine. Neuropsychopharmacology 38, 2688–2697 (2013).
Gisabella, B., Scammell, T., Bandaru, S. S. & Saper, C. B. Regulation of hippocampal dendritic spines following sleep deprivation. J. Comp. Neurol. 528, 380–388 (2020).
Bolsius, Y. G., Meerlo, P., Kas, M. J., Abel, T. & Havekes, R. Sleep deprivation reduces the density of individual spine subtypes in a branch-specific fashion in CA1 neurons. J. Sleep Res. 31, e13438 (2022).
Varela, C. & Wilson, M. A. mPFC spindle cycles organize sparse thalamic activation and recently active CA1 cells during non-REM sleep. eLife 9, e48881 (2020).
Mavanji, V. & Datta, S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur. J. Neurosci. 17, 359–370 (2003).
Tsunematsu, T., Patel, A. A., Onken, A. & Sakata, S. State-dependent brainstem ensemble dynamics and their interactions with hippocampus across sleep states. eLife 9, e52244 (2020).
Shin, J. N., Doron, G. & Larkum, M. E. Memories off the top of your head. Science 374, 538–539 (2021).
Palacios-Filardo, J. & Mellor, J. R. Neuromodulation of hippocampal long-term synaptic plasticity. Curr. Opin. Neurobiol. 54, 37–43 (2019).
Nakauchi, S., Brennan, R. J., Boulter, J. & Sumikawa, K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur. J. Neurosci. 25, 2666–2681 (2007).
Delorme, J. et al. Sleep loss drives acetylcholine- and somatostatin interneuron-mediated gating of hippocampal activity to inhibit memory consolidation. Proc. Natl Acad. Sci. USA 118, e2019318118 (2021).
Soulé, J. et al. Balancing Arc synthesis, mRNA decay, and proteasomal degradation: maximal protein expression triggered by rapid eye movement sleep-like bursts of muscarinic cholinergic receptor stimulation. J. Biol. Chem. 287, 22354–22366 (2012).
Stefanelli, T., Bertollini, C., Lüscher, C., Muller, D. & Mendez, P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89, 1074–1085 (2016).
Dannenberg, H., Young, K. & Hasselmo, M. Modulation of hippocampal circuits by muscarinic and nicotinic receptors. Front. Neural Circuits 11, 102 (2017).
Rexrode, L. et al. Regulation of dendritic spines in the amygdala following sleep deprivation. Front. Sleep 2, 1145203 (2023).
Van Someren, E. J. W. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol. Rev. 101, 995–1046 (2021).
Bröcher, S., Artola, A. & Singer, W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 573, 27–36 (1992).
Suzuki, A., Yanagisawa, M. & Greene, R. W. Loss of Arc attenuates the behavioral and molecular responses for sleep homeostasis in mice. Proc. Natl Acad. Sci. USA 117, 10547–10553 (2020).
Poe, G. R., Nitz, D. A., McNaughton, B. L. & Barnes, C. A. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000).
Ramirez-Villegas, J. F. et al. Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nature 589, 96–102 (2021).
Booth, V. & Poe, G. R. Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: relevance to REM sleep reactivation and memory consolidation. Hippocampus 16, 161–173 (2006). This paper shows how the consolidation process of strengthening the familiarity-encoding temporoammonic distal hippocampal dendrites can cause CA1 neurons to fire at proximal theta troughs which, through spike timing-dependent plasticity, invokes depotentiation at novelty-encoding proximal dendrites.
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Bi, G. Q. & Poo, M. M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 18, 10464–10472 (1998).
Grella, S. L. et al. Locus coeruleus phasic, but not tonic, activation initiates global remapping in a familiar environment. J. Neurosci. 39, 445–455 (2019).
Andrade, K. C. et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J. Neurosci. 31, 10331–10339 (2011).
Buzsáki, G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015).
Cox, R., Rüber, T., Staresina, B. P. & Fell, J. Sharp wave-ripples in human amygdala and their coordination with hippocampus during NREM sleep. Cereb. Cortex Commun. 1, tgaa051 (2020).
Kim, W. B. & Cho, J.-H. Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J. Neurosci. 37, 4868–4882 (2017).
Li, W., Ma, L., Yang, G. & Gan, W.-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 20, 427–437 (2017).
Zhou, Y. et al. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat. Commun. 11, 4819 (2020). This paper demonstrates that experience-dependent structural plasticity takes place during REM sleep in cortical neurons.
Popa, D., Duvarci, S., Popescu, A. T., Léna, C. & Paré, D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl Acad. Sci. USA 107, 6516–6519 (2010).
Gais, S. et al. Sleep transforms the cerebral trace of declarative memories. Proc. Natl Acad. Sci. USA 104, 18778–18783 (2007).
Sterpenich, V. et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PloS Biol. 5, e282 (2007).
van der Helm, E. et al. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr. Biol. 21, 2029–2032 (2011).
Motomura, Y. et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PloS ONE 8, e56578 (2013).
Sollenberger, N. A. et al. Sleep fails to depotentiate amygdala-reactivity to negative emotional stimuli in youth with elevated symptoms of anxiety. Cogn. Affect. Behav. Neurosci. 23, 415–426 (2023).
Sterpenich, V. et al. Sleep promotes the neural reorganization of remote emotional memory. J. Neurosci. 29, 5143–5152 (2009).
Yoo, S.-S., Gujar, N., Hu, P., Jolesz, F. A. & Walker, M. P. The human emotional brain without sleep — a prefrontal amygdala disconnect. Curr. Biol. 17, R877–R878 (2007).
Nowak, J. et al. Association of naturally occurring sleep loss with reduced amygdala resting-state functional connectivity following psychosocial stress. Psychoneuroendocrinology 114, 104585 (2020).
Gilson, M. et al. REM-enriched naps are associated with memory consolidation for sad stories and enhance mood-related reactivity. Brain Sci. 6, 1 (2015).
Werner, G. G., Schabus, M., Blechert, J., Kolodyazhniy, V. & Wilhelm, F. H. Pre- to postsleep change in psychophysiological reactivity to emotional films: late-night REM sleep is associated with attenuated emotional processing. Psychophysiology 52, 813–825 (2015).
Cunningham, T. J. et al. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiol. Learn. Mem. 114, 155–164 (2014).
Reinhold, F. L., Gerlicher, A. M. V., van Someren, E. J. W. & Kindt, M. Do your troubles today seem further away than yesterday? On sleep’s role in mitigating the blushing response to a reactivated embarrassing episode. Sleep 45, zsac220 (2022).
Jones, B. J. & Spencer, R. M. C. Sleep preserves subjective and sympathetic emotional response of memories. Neurobiol. Learn. Mem. 166, 107096 (2019).
Pace-Schott, E. F. et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol. Learn. Mem. 95, 24–36 (2011).
Werner, G. G., Schabus, M., Blechert, J. & Wilhelm, F. H. Differential effects of REM sleep on emotional processing: initial evidence for increased short-term emotional responses and reduced long-term intrusive memories. Behav. Sleep Med. 19, 83–98 (2021).
Pesonen, A.-K. et al. Presleep physiological stress is associated with a higher cortical arousal in sleep and more consolidated REM sleep. Stress 24, 667–675 (2021).
Spoormaker, V. I., Gvozdanovic, G. A., Sämann, P. G. & Czisch, M. Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Exp. Brain Res. 232, 1547–1554 (2014).
van Marle, H. J. F., Hermans, E. J., Qin, S., Overeem, S. & Fernández, G. The effect of exogenous cortisol during sleep on the behavioral and neural correlates of emotional memory consolidation in humans. Psychoneuroendocrinology 38, 1639–1649 (2013).
Beck, J., Loretz, E. & Rasch, B. Stress dynamically reduces sleep depth: temporal proximity to the stressor is crucial. Cereb. Cortex 33, 96–113 (2022).
Riemann, D. et al. REM sleep instability — a new pathway for insomnia? Pharmacopsychiatry 45, 167–176 (2012). This paper first describes REM sleep instability in insomnia as a transdiagnostic risk factor for disturbed emotion regulation and mental health problems.
Germain, A. Sleep disturbances as the hallmark of PTSD: where are we now? Am. J. Psychiatry 170, 372–382 (2013).
Pesonen, A.-K. et al. REM sleep fragmentation associated with depressive symptoms and genetic risk for depression in a community-based sample of adolescents. J. Affect. Disord. 245, 757–763 (2019).
Galbiati, A. et al. The association between emotional dysregulation and REM sleep features in insomnia disorder. Brain Cogn. 146, 105642 (2020).
Halonen, R., Kuula, L., Makkonen, T., Kauramäki, J. & Pesonen, A.-K. Self-conscious affect is modulated by rapid eye movement sleep but not by targeted memory reactivation — a pilot study. Front. Psychol. 12, 730924 (2021).
Bottary, R. et al. Fear extinction memory is negatively associated with REM sleep in insomnia disorder. Sleep 43, zsaa007 (2020).
Seo, J. et al. Delayed fear extinction in individuals with insomnia disorder. Sleep 41, zsy095 (2018).
Insana, S. P., Kolko, D. J. & Germain, A. Early-life trauma is associated with rapid eye movement sleep fragmentation among military veterans. Biol. Psychol. 89, 570–579 (2012).
Mellman, T. A., Bustamante, V., Fins, A. I., Pigeon, W. R. & Nolan, B. REM sleep and the early development of posttraumatic stress disorder. Am. J. Psychiatry 159, 1696–1701 (2002).
Mellman, T. A., Pigeon, W. R., Nowell, P. D. & Nolan, B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J. Trauma. Stress. 20, 893–901 (2007).
Cowdin, N., Kobayashi, I. & Mellman, T. A. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp. Brain Res. 232, 1479–1485 (2014).
Gong, L. et al. The abnormal functional connectivity in the locus coeruleus-norepinephrine system associated with anxiety symptom in chronic insomnia disorder. Front. Neurosci. 15, 678465 (2021). This paper has found direct evidence for the links between altered functional connectivity between the LC and salience network in insomnia and clinically relevant anxiety.
Talamini, L. M., Bringmann, L. F., de Boer, M. & Hofman, W. F. Sleeping worries away or worrying away sleep? Physiological evidence on sleep-emotion interactions. PloS ONE 8, e62480 (2013).
Cellini, N., Mercurio, M. & Sarlo, M. The fate of emotional memories over a week: does sleep play any role? Front. Psychol. 10, 481 (2019).
Gujar, N., McDonald, S. A., Nishida, M. & Walker, M. P. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb. Cortex 21, 115–123 (2011).
Kleim, B. et al. Sleep enhances exposure therapy. Psychol. Med. 44, 1511–1519 (2014).
Wassing, R., Benjamins, J. S., Talamini, L. M., Schalkwijk, F. & Van Someren, E. J. W. Overnight worsening of emotional distress indicates maladaptive sleep in insomnia. Sleep 42, zsz051 (2019).
Baran, B., Pace-Schott, E. F., Ericson, C. & Spencer, R. M. C. Processing of emotional reactivity and emotional memory over sleep. J. Neurosci. 32, 1035–1042 (2012).
Tempesta, D., De Gennaro, L., Natale, V. & Ferrara, M. Emotional memory processing is influenced by sleep quality. Sleep. Med. 16, 862–870 (2015).
Harrington, M. O., Nedberge, K. M. & Durrant, S. J. The effect of sleep deprivation on emotional memory consolidation in participants reporting depressive symptoms. Neurobiol. Learn. Mem. 152, 10–19 (2018).
Lau, E. Y. Y. et al. Effects of REM sleep during a daytime nap on emotional perception in individuals with and without depression. J. Affect. Disord. 260, 687–694 (2020).
Zeng, S., Lin, X., Wang, J. & Hu, X. Sleep’s short-term memory preservation and long-term affect depotentiation effect in emotional memory consolidation: behavioral and EEG evidence. Sleep 44, zsab155 (2021).
Davidson, P. & Pace-Schott, E. Go to bed and you MIGHT feel better in the morning — the effect of sleep on affective tone and intrusiveness of emotional memories. Curr. Sleep Med. Rep. 7, 31–46 (2021).
Etkin, A., Büchel, C. & Gross, J. J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693–700 (2015).
Wiesner, C. D. et al. The effect of selective REM-sleep deprivation on the consolidation and affective evaluation of emotional memories. Neurobiol. Learn. Mem. 122, 131–141 (2015).
Wagner, U., Fischer, S. & Born, J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom. Med. 64, 627–634 (2002).
Lara-Carrasco, J., Nielsen, T. A., Solomonova, E., Levrier, K. & Popova, A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. J. Sleep Res. 18, 178–187 (2009).
Greenberg, R., Pillard, R. & Pearlman, C. The effect of dream (stage REM) deprivation on adaptation to stress. Psychosom. Med. 34, 257–262 (1972).
Rosales-Lagarde, A. et al. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Front. Behav. Neurosci. 6, 25 (2012).
Llewellyn, S. & Hobson, J. A. Not only … but also: REM sleep creates and NREM stage 2 instantiates landmark junctions in cortical memory networks. Neurobiol. Learn. Mem. 122, 69–87 (2015).
Cairney, S. A., Durrant, S. J., Power, R. & Lewis, P. A. Complementary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation. Cereb. Cortex 25, 1565–1575 (2015).
Rihm, J. S. & Rasch, B. Replay of conditioned stimuli during late REM and stage N2 sleep influences affective tone rather than emotional memory strength. Neurobiol. Learn. Mem. 122, 142–151 (2015).
Hutchison, I. C. et al. Targeted memory reactivation in REM but not SWS selectively reduces arousal responses. Commun. Biol. 4, 404 (2021). This paper shows that targeted memory reactivation during REM sleep enhances overnight adaptation in subjective arousal ratings to multisensory emotional stimuli.
Xia, T. et al. Updating memories of unwanted emotions during human sleep. Curr. Biol. 33, 309–320.e5 (2023).
Groch, S. et al. Targeted reactivation during sleep differentially affects negative memories in socially anxious and healthy children and adolescents. J. Neurosci. 37, 2425–2434 (2017).
Pereira, S. I. R. et al. Cueing emotional memories during slow wave sleep modulates next-day activity in the orbitofrontal cortex and the amygdala. NeuroImage 253, 119120 (2022).
Borghese, F. et al. Targeted memory reactivation during REM sleep in patients with social anxiety disorder. Front. Psychiatry 13, 904704 (2022).
Hauner, K. K., Howard, J. D., Zelano, C. & Gottfried, J. A. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat. Neurosci. 16, 1553–1555 (2013).
Spencer, R. L. & Bland, S. T. in Stress: Physiology, Biochemistry, and Pathology (ed. Fink, G.) 57–68 (Academic, 2019).
Acknowledgements
Y.C. discloses support for this work from the Cota Robles Fellowship. G.R.P. discloses support from the National Institute of Mental Health (R01 Grant MH60670). H.W.K. discloses support from the Brain Foundation Netherlands (DR-2018-00252). E.J.W.V.S. discloses support from the European Research Council (ERC-ADG-2014-671084 INSOMNIA and ERC-2021-ADG-101055383 OVERNIGHT) and from the Netherlands Organisation for Health Research and Development (ZonMw) project REMOVE 09120011910032. R.W. discloses support from the Sydney Local Health District and the Australian National Health and Medical Research Council (Investigator Grant GNT1196636).
Author information
Authors and Affiliations
Contributions
All authors drafted, edited and revised the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Anu-Katriina Pesonen and Julie Seibt for their contribution to the peer review of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cabrera, Y., Koymans, K.J., Poe, G.R. et al. Overnight neuronal plasticity and adaptation to emotional distress. Nat. Rev. Neurosci. 25, 253–271 (2024). https://doi.org/10.1038/s41583-024-00799-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-024-00799-w