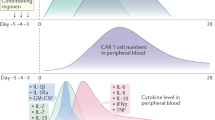
Abstract
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of several haematological malignancies and is being investigated in patients with various solid tumours. Characteristic CAR T cell-associated toxicities such as cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are now well-recognized, and improved supportive care and management with immunosuppressive agents has made CAR T cell therapy safer and more feasible than it was when the first regulatory approvals of such treatments were granted in 2017. The increasing clinical experience with these therapies has also improved recognition of previously less well-defined toxicities, including movement disorders, immune effector cell-associated haematotoxicity (ICAHT) and immune effector cell-associated haemophagocytic lymphohistiocytosis-like syndrome (IEC-HS), as well as the substantial risk of infection in patients with persistent CAR T cell-induced B cell aplasia and hypogammaglobulinaemia. A more diverse selection of immunosuppressive and supportive-care pharmacotherapies is now being utilized for toxicity management, yet no universal algorithm for their application exists. As CAR T cell products targeting new antigens are developed, additional toxicities involving damage to non-malignant tissues expressing the target antigen are a potential hurdle. Continued prospective evaluation of toxicity management strategies and the design of less-toxic CAR T cell products are both crucial for ongoing success in this field. In this Review, we discuss the evolving understanding and clinical management of CAR T cell-associated toxicities.
Key points
-
As clinicians have gained more experience with chimeric antigen receptor (CAR) T cell therapy, the management of cytokine-release syndrome (CRS) has improved, especially with use of the IL-6 receptor antagonist tocilizumab.
-
In addition, monitoring and treatment of immune effector cell-associated neurotoxicity syndrome (ICANS) has improved with refinements to supportive care and glucocorticoid use.
-
Movement disorders are an infrequent complication of B cell maturation antigen (BCMA)-directed CAR T cells, are difficult to manage and can be life-threatening.
-
Prolonged cytopenias, secondary haemophagocytic lymphohistiocytosis and infectious complications are increasingly well recognized, and consensus guidelines have been developed for their management.
-
New T cell malignancies have been reported after CAR T cell therapy but are exceedingly rare. Patients should be monitored for second malignancies indefinitely following CAR T cell therapy.
-
Future directions in this field include the development of less-toxic CAR T cell products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Fowler, N. H. et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat. Med. 28, 325–332 (2022).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Jacobson, C. A. et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 23, 91–103 (2022).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Siddiqi, T. et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1-2 study. Lancet 402, 641–654 (2023).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Shah, B. D. et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Mailankody, S. et al. GPRC5D-targeted CAR T cells for myeloma. N. Engl. J. Med. 387, 1196–1206 (2022).
Zhang, M. et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 10, e107–e116 (2023).
Olson, D. J. & Odunsi, K. Adoptive cell therapy for nonhematologic solid tumors. J. Clin. Oncol. 41, 3397–3407 (2023).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
DeAngelo, D. J. et al. Clinical outcomes for the phase 2, single-arm, multicenter trial of JCAR015 in adult B-ALL (ROCKET study) [abstract 217]. J. Immunother. Cancer, 5 (Suppl. 2), 86 (2017).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Reagan, P. M. & Neelapu, S. S. How I manage: pathophysiology and management of toxicity of chimeric antigen receptor T-cell therapies. J. Clin. Oncol. 39, 456–466 (2021).
Jacobson, C. A. et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J. Clin. Oncol. 38, 3095–3106 (2020).
Hansen, D. K. et al. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: real-world experience from the Myeloma CAR T Consortium. J. Clin. Oncol. 41, 2087–2097 (2023).
Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306 (2017).
Maus, M. V. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Locke, F. L. et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 4, 4898–4911 (2020).
Brudno, J. N. et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36, 2267–2280 (2018).
Zhou, L. et al. Derivation and validation of a novel score for early prediction of severe CRS after CAR-T therapy in haematological malignancy patients: a multi-centre study. Br. J. Haematol. 202, 517–524 (2023).
Greenbaum, U. et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv. 5, 2799–2806 (2021).
Pennisi, M. et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 5, 3397–3406 (2021).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Gust, J. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Brudno, J. N. & Kochenderfer, J. N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 34, 45–55 (2019).
Locke, F. L. et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL) [abstract]. Blood 130 (Suppl. 1), 1547 (2017).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Strati, P. et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood 137, 3272–3276 (2021).
Nastoupil, L. J. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 38, 3119–3128 (2020).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Trans. Med. 5, 177ra38 (2013).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Trans. Med. 3, 95ra73 (2011).
Duvalyan, E. et al. Impact of corticosteroids on efficacy of BCMA targeted CAR-T therapy in multiple myeloma. Leuk. Lymphoma 64, 1888–1891 (2023).
Santomasso, B. D. et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 39, 3978–3992 (2021).
Hayden, P. J. et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 33, 259–275 (2022).
NCCN. NCCN Guidelines. Management of Immunotherapy-Related Toxicities (version 1.2024). nccn.org, https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1486 (2024).
Boyle, S. et al. Improved outcomes of large B-cell lymphoma patients treated with CD19 CAR T in the UK over time. Br. J. Haematol. 204, 507–513 (2023).
Gardner, R. A. et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 134, 2149–2158 (2019).
Caimi, P. F. et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front. Immunol. 12, 745320 (2021).
Topp, M. S. et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br. J. Haematol. 195, 388–398 (2021).
Oluwole, O. O. et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br. J. Haematol. 194, 690–700 (2021).
Kadauke, S. et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J. Clin. Oncol. 39, 920–930 (2021).
Gauthier, J. et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 135, 1650–1660 (2020).
Gill, S. I. et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 6, 5774–5785 (2022).
Frigault, M. J. et al. Itacitinib for the prevention of immune effector cell therapy-associated cytokine release syndrome: results from the phase 2 Incb 39110-211 placebo-controlled randomized cohort [abstract]. Blood 142 (Suppl. 1), 356 (2023).
Gutierrez, C. et al. The chimeric antigen receptor-intensive care unit (CAR-ICU) initiative: surveying intensive care unit practices in the management of CAR T-cell associated toxicities. J. Crit. Care 58, 58–64 (2020).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Strati, P. et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020).
Jatiani, S. S. et al. Myeloma CAR-T CRS management with IL-1R antagonist anakinra. Clin. Lymphoma Myeloma Leuk. 20, 632–636.e1 (2020).
Diorio, C. et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv. 6, 3398–3403 (2022).
Pan, J. et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J. Cell. Mol. Med. 25, 1089–1099 (2021).
McNerney, K. O., DiNofia, A. M., Teachey, D. T., Grupp, S. A. & Maude, S. L. Potential role of IFNγ inhibition in refractory cytokine release syndrome associated with CAR T-cell therapy. Blood Cancer Discov. 3, 90–94 (2022).
Bailey, S. R. et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov. 3, 136–153 (2022).
Mestermann, K. et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 11, eaau5907 (2019).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Baur, K. et al. Dasatinib for treatment of CAR T-cell therapy-related complications. J. Immunother. Cancer 10, e005956 (2022).
Mikkilineni, L. et al. Rapid anti-myeloma activity by T cells expressing an anti-BCMA CAR with a human heavy-chain-only antigen-binding domain. Mol. Ther. 32, 503–526 (2024).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Brudno, J. N. et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 26, 270–280 (2020).
Taraseviciute, A. et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 8, 750–763 (2018).
Saini, N. Y. et al. Clonal hematopoiesis is associated with increased risk of severe neurotoxicity in axicabtagene ciloleucel therapy of large B-cell lymphoma. Blood Cancer Discov. 3, 385–393 (2022).
Bachy, E. et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 28, 2145–2154 (2022).
Cancer Therapy Evaluation Program. Common terminology criteria for adverse events (CTCAE). version 4.03. CTEP https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (2010).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Shalabi, H. et al. Intrathecal hydrocortisone for treatment of children and young adults with CAR T-cell immune-effector cell-associated neurotoxicity syndrome. Pediatr. Blood Cancer 71, e30741 (2024).
Zurko, J. C. et al. Use of early intrathecal therapy to manage high-grade immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. 8, 773–775, (2022).
Park, J. H. et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat. Med. 29, 1710–1717 (2023).
Wehrli, M. et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 10, e003847 (2022).
Martin, T. et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 41, 1265–1274 (2023).
Cohen, A. D. et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 12, 32 (2022).
Karschnia, P. et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 142, 1243–1248 (2023).
Van Oekelen, O. et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 27, 2099–2103 (2021).
Marella, M. et al. Comprehensive BCMA expression profiling in adult normal human brain suggests a low risk of on-target neurotoxicity in BCMA-targeting multiple myeloma therapy. J. Histochem. Cytochem. 70, 273–287 (2022).
Graham, C. E. et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel-associated movement and neurocognitive toxicity. Blood 142, 1248–1252 (2023).
Jain, T., Olson, T. S. & Locke, F. L. How I treat cytopenias after CAR T-cell therapy. Blood 141, 2460–2469 (2023).
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 20, 31–42 (2019).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Iacoboni, G. et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 6, 3606–3610 (2022).
Westin, J. R. et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. 389, 148–157 (2023).
Ramos, C. A. et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 38, 3794–3804 (2020).
Brudno, J. N. et al. Transient responses and significant toxicities of anti-CD30 CAR T cells for CD30+ lymphomas: results of a phase I trial. Blood Adv. 8, 802–814 (2023).
Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med. 29, 2844–2853 (2023).
Fried, S. et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 54, 1643–1650 (2019).
Brudno, J. N. et al. Acute and delayed cytopenias following CAR T-cell therapy: an investigation of risk factors and mechanisms. Leuk. Lymphoma 63, 1849–1860 (2022).
Rejeski, K. et al. Severe hematotoxicity after CD19 CAR-T therapy is associated with suppressive immune dysregulation and limited CAR-T expansion. Sci. Adv. 9, eadg3919 (2023).
Jain, T. et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 4, 3776–3787 (2020).
Brudno, J. N. et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 34, 1112–1121 (2016).
Rejeski, K. et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood 138, 2499–2513 (2021).
Juluri, K. R. et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 6, 2055–2068 (2022).
Nagle, S. J. et al. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am. J. Hematol. 96, 455–461 (2021).
Kitamura, W. et al. Bone marrow microenvironment disruption and sustained inflammation with prolonged haematologic toxicity after CAR T-cell therapy. Br. J. Haematol. 202, 294–307 (2023).
Strati, P. et al. Prolonged cytopenia following CD19 CAR T cell therapy is linked with bone marrow infiltration of clonally expanded IFNγ-expressing CD8 T cells. Cell Rep. Med. 4, 101158 (2023).
Rejeski, K. et al. An international survey on grading, diagnosis, and management of immune effector cell-associated hematotoxicity (ICAHT) following CAR T-cell therapy on behalf of the EBMT and EHA. Hemasphere 7, e889 (2023).
Rejeski, K. et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood 142, 865–877 (2023).
Rejeski, K. et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 10, e004475 (2022).
Rejeski, K. et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J. Hematol. Oncol. 16, 88 (2023).
Rejeski, K. et al. The CAR-HEMATOTOX score identifies patients at high risk for hematological toxicity, infectious complications, and poor treatment outcomes following brexucabtagene autoleucel for relapsed or refractory MCL. Am. J. Hematol. 98, 1699–1710 (2023).
Galli, E. et al. G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transpl. 55, 2347–2349 (2020).
Cao, M. et al. Early granulocyte colony stimulating factor administration increases the risk of cytokine release syndrome in acute lymphoblastic leukemia patients receiving anti-CD19 chimeric antigen receptor T-cell therapy. Hematol. Oncol. 41, 933–941 (2023).
Miller, K. C. et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 12, 146 (2022).
Alvarado, L. J. et al. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood 133, 2043–2055 (2019).
Drillet, G., Lhomme, F., De Guibert, S., Manson, G. & Houot, R. Prolonged thrombocytopenia after CAR T-cell therapy: the role of thrombopoietin receptor agonists. Blood Adv. 7, 537–540 (2023).
Wang, J. et al. Low-dose administration of prednisone has a good effect on the treatment of prolonged hematologic toxicity post-CD19 CAR-T cell therapy. Front. Immunol. 14, 1139559 (2023).
Gagelmann, N. et al. Hematopoietic stem cell boost for persistent neutropenia after CAR-T cell therapy: a GLA/DRST study. Blood Adv. 7, 555–559 (2020).
Patwari, A. et al. The effect of stem cell infusion on immune effector cell associated hematotoxicity with BCMA CAR T in multiple myeloma [abstract]. Blood 142 (Suppl. 1), 758 (2023).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Lichtenstein, D. A. et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood 138, 2469–2484 (2021).
Hines, M. R. et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl. Cell Ther. 29, 438.e1–438.e16 (2023).
Neelapu, S. S. et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141, 2307–2315 (2023).
Chong, E. A., Ruella, M. & Schuster, S. J. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N. Engl. J. Med. 384, 673–674 (2021).
Jarisch, A. et al. Immune responses to SARS-CoV-2 vaccination in young patients with anti-CD19 chimeric antigen receptor T cell-induced B cell aplasia. Transpl. Cell Ther. 28, 366.e1–366.e7 (2022).
Pasquini, M. C. et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 4, 5414–5424 (2020).
Cappell, K. M. et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J. Clin. Oncol. 38, 3805–3815 (2020).
Bhoj, V. G. et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 128, 360–370 (2016).
Hill, J. A. et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv. 3, 3590–3601 (2019).
Lee, D. et al. Pneumococcal conjugate vaccine does not induce humoral response when administrated within the six months after CD19 CAR T-cell therapy. Transpl. Cell Ther. 29, 277.e1–277.e9 (2023).
Kampouri, E., Walti, C. S., Gauthier, J. & Hill, J. A. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert. Rev. Hematol. 15, 305–320 (2022).
Kampouri, E. et al. Infections after chimeric antigen receptor (CAR)-T-cell therapy for hematologic malignancies. Transpl. Infect. Dis. 25, e14157 (2023).
Lancman, G. et al. IVIg use associated with ten-fold reduction of serious infections in multiple myeloma patients treated with anti-BCMA bispecific antibodies. Blood Cancer Discov. 4, 440–451 (2023).
Logue, J. M. et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 106, 978–986 (2021).
Frigault, M. J. et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood 136, 137–139 (2020).
Benjamin, R. et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet Haematol. 9, e833–e843 (2022).
Cornetta, K. et al. Replication competent retrovirus testing (RCR) in the National Gene Vector Biorepository: no evidence of RCR in 1,595 post-treatment peripheral blood samples obtained from 60 clinical trials. Mol. Ther. 31, 801–809 (2023).
Kampouri, E., Hill, J. A. & Dioverti, V. COVID-19 after hematopoietic cell transplantation and chimeric antigen receptor (CAR)-T-cell therapy. Transpl. Infect. Dis. 25, e14144 (2023).
Infante, M. S. et al. Outcomes and management of the SARS-CoV2 omicron variant in recipients of hematopoietic cell transplantation and chimeric antigen receptor T cell therapy. Transpl. Cell Ther. 30, 116.e1–116.e12 (2023).
van Doesum, J. A. et al. Impact of SARS-CoV-2 vaccination and monoclonal antibodies on outcome post-CD19-directed CAR T-cell therapy: an EPICOVIDEHA survey. Blood Adv. 7, 2645–2655 (2023).
Nussenblatt, V. et al. Yearlong COVID-19 infection reveals within-host evolution of SARS-CoV-2 in a patient with B-cell depletion. J. Infect. Dis. 225, 1118–1123 (2022).
Aleissa, M. M. et al. Severe acute respiratory syndrome coronavirus 2 vaccine immunogenicity among chimeric antigen receptor T cell therapy recipients. Transpl. Cell Ther. 29, 398.e1–398.e5 (2023).
Wiedmeier-Nutor, J. E. et al. Response to COVID-19 vaccination post-CAR T therapy in patients with non-hodgkin lymphoma and multiple myeloma. Clin. Lymphoma Myeloma Leuk. 23, 456–462 (2023).
Inoue, S., Nambu, T. & Shimomura, T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J. Invest. Dermatol. 122, 565–573 (2004).
Gagelmann, N. & Brudno, J. GPRC5D-targeting chimeric antigen receptors: a new treatment for multiple myeloma? Lancet Haematol. 10, e82–e83 (2023).
Atilla, E. & Benabdellah, K. The black hole: CAR T cell therapy in AML. Cancers 15, 2713 (2023).
Haas, A. R. et al. Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells. Mol. Ther. 31, 2309–2325 (2023).
Ghilardi, G. et al. T-cell lymphoma and secondary primary malignancy risk after commercial CAR T-cell therapy. Nat. Med. 30, 984–989 (2024).
Legend Biotech Corporation. Legend Biotech Announces U.S. FDA Label Update for CARVYKTI® (ciltacabtagene autoleucel; cilta-cel). Legend Biotech Corporation https:// investors.legendbiotech.com/static-files/e40632fa-bb0c-4a3e-ac55-0ca85b212d76 (2023).
Maclachlan, K. et al. Second malignancies in multiple myeloma; emerging patterns and future directions. Best. Pract. Res. Clin. Haematol. 33, 101144 (2020).
US Food and Drug Administration. FDA Investigating Serious Risk of T-cell Malignancy Following BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies. FDA https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous (2023).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Micklethwaite, K. P. et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 138, 1391–1405 (2021).
Bishop, D. C. et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood 138, 1504–1509 (2021).
Harrison, S. J. et al. CAR+ T-cell lymphoma post ciltacabtagene autoleucel therapy for relapsed refractory multiple myeloma [abstract]. Blood 142 (Suppl. 1), 6939 (2023).
Chihara, D., Dores, G. M., Flowers, C. R. & Morton, L. M. The bidirectional increased risk of B-cell lymphoma and T-cell lymphoma. Blood 138, 785–789 (2021).
Trab, T. et al. Second primary malignancies in patients with lymphoma in Denmark after high-dose chemotherapy and autologous haematopoietic stem-cell transplantation: a population-based, retrospective cohort study. Lancet Haematol. 10, e838–e848 (2023).
Kalaycio, M. et al. Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia. J. Clin. Oncol. 24, 3604–3610 (2006).
Levine, B. L. et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat. Med. 30, 338–341 (2024).
Roddie, C. et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J. Clin. Oncol. 39, 3352–3363 (2021).
Cappell, K. M. & Kochenderfer, J. N. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 18, 715–727 (2021).
Alabanza, L. et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol. Ther. 25, 2452–2465 (2017).
Weinkove, R. et al. A phase 1 dose escalation trial of third-generation CD19-directed CAR T-cells incorporating CD28 and toll-like receptor 2 (TLR2) intracellular domains for relapsed or refractory B-cell non-Hodgkin lymphomas (ENABLE) [abstract]. Blood 142 (Suppl. 1), 890 (2023).
Park, J. H. et al. A phase I study of CD19-targeted 19(T2)28z1xx CAR T cells in adult patients with relapsed or refractory diffuse large B-cell lymphoma [abstract]. Blood 142 (Suppl. 1), 892 (2023).
Foster, M. C. et al. Utility of a safety switch to abrogate CD19.CAR T-cell-associated neurotoxicity. Blood 137, 3306–3309 (2021).
Xue, L. et al. Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy. Cell Discov. 7, 84 (2021).
Aldoss, I. et al. Favorable activity and safety profile of memory-enriched CD19-targeted chimeric antigen receptor T-cell therapy in adults with high-risk relapsed/refractory ALL. Clin. Cancer Res. 29, 742–753 (2023).
Vander Mause, E. R. et al. Systematic single amino acid affinity tuning of CD229 CAR T cells retains efficacy against multiple myeloma and eliminates on-target off-tumor toxicity. Sci. Transl. Med. 15, eadd7900 (2023).
Hamieh, M., Mansilla-Soto, J., Riviere, I. & Sadelain, M. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov. 13, 829–843 (2023).
Bangayan, N. J. et al. Dual-inhibitory domain iCARs improve the efficiency of the AND-` CAR T strategy. Proc. Natl Acad. Sci. USA 120, e2312374120 (2023).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29, 422–429 (2023).
Chiesa, R. et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N. Engl. J. Med. 389, 899–910 (2023).
Lu, P. et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood 140, 321–334 (2022).
Schuster, S. J. et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 4, 1432–1439 (2020).
Abramson, J. S. et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood 141, 1675–1684 (2023).
Laetsch, T. W. et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J. Clin. Oncol. 41, 1664–1669 (2023).
Siddiqi, T. et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 139, 1794–1806 (2021).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Gutierrez, C., Neilan, T. G. & Grover, N. S. How I approach optimization of patients at risk of cardiac and pulmonary complications after CAR T-cell therapy. Blood 141, 2452–2459 (2023).
Bachanova, V. et al. Chimeric antigen receptor T cell therapy during the COVID-19 pandemic. Biol. Blood Marrow Transpl. 26, 1239–1246 (2020).
Reynolds, G., Hall, V. G. & Teh, B. W. Vaccine schedule recommendations and updates for patients with hematologic malignancy post-hematopoietic cell transplant or CAR T-cell therapy. Transpl. Infect. Dis. 25 (suppl. 1), e14109 (2023).
Dioverti, V. et al. Revised guidelines for coronavirus disease 19 management in hematopoietic cell transplantation and cellular therapy recipients (August 2022). Transpl. Cell Ther. 28, 810–821 (2022).
Acknowledgements
The authors acknowledge intramural National Cancer Institute (NCI) funding support.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.N.B. serves on the scientific advisory board of Kyverna Therapeutics (unpaid position). J.N.K. receives royalties from Kite Pharma (a Gilead company), Celgene/Bristol Myers Squibb and Kyverna Therapeutics; and research funding from Kite (a Gilead company) and Celgene/Bristol Myers Squibb.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S. Mailankody, who co-reviewed with E. Jurgens; D. Lee; F. Locke; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brudno, J.N., Kochenderfer, J.N. Current understanding and management of CAR T cell-associated toxicities. Nat Rev Clin Oncol (2024). https://doi.org/10.1038/s41571-024-00903-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41571-024-00903-0