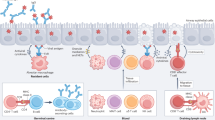
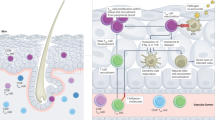
Abstract
Vaccination remains our main defence against influenza, which causes substantial annual mortality and poses a serious pandemic threat. Influenza virus evades immunity by rapidly changing its surface antigens but, even when the vaccine is well matched to the current circulating virus strains, influenza vaccines are not as effective as many other vaccines. Influenza vaccine development has traditionally focused on the induction of protective antibodies, but there is mounting evidence that T cell responses are also protective against influenza. Thus, future vaccines designed to promote both broad T cell effector functions and antibodies may provide enhanced protection. As we discuss, such vaccines present several challenges that require new strategic and economic considerations. Vaccine-induced T cells relevant to protection may reside in the lungs or lymphoid tissues, requiring more invasive assays to assess the immunogenicity of vaccine candidates. T cell functions may contain and resolve infection rather than completely prevent infection and early illness, requiring vaccine effectiveness to be assessed based on the prevention of severe disease and death rather than symptomatic infection. It can be complex and costly to measure T cell responses and infrequent clinical outcomes, and thus innovations in clinical trial design are needed for economic reasons. Nevertheless, the goal of more effective influenza vaccines justifies renewed and intensive efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Potter, C. W. A history of influenza. J. Appl. Microbiol. 91, 572–579 (2001).
Creighton, C. A History of Epidemics in Britain from A.D. 664 to the Extinction of Plague (Cambridge Univ. Press, 1891).
Dowdle, W. R. Influenza A virus recycling revisited. Bull. World Health Organ. 77, 820–828 (1999).
Taubenberger, J. K., Reid, A. H., Krafft, A. E., Bijwaard, K. E. & Fanning, T. G. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 275, 1793–1796 (1997).
Iuliano, A. D. et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300 (2018).
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2021–2022 northern hemisphere influenza season. Wkly. Epidemiological Rec. 96, 77–88 (2021).
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022–2023 northern hemisphere influenza season. Wkly Epidemiological Rec. 97, 109–132 (2022).
Jackson, L. A. Using surveillance to evaluate influenza vaccine effectiveness. J. Infect. Dis. 199, 155–158 (2009).
Jackson, M. L. & Nelson, J. C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 31, 2165–2168 (2013).
Sullivan, S. G., Tchetgen Tchetgen, E. J. & Cowling, B. J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am. J. Epidemiol. 184, 345–353 (2016).
Feng, S., Cowling, B. J., Kelly, H. & Sullivan, S. G. Estimating influenza vaccine effectiveness with the test-negative design using alternative control groups: a systematic review and meta-analysis. Am. J. Epidemiol. 187, 389–397 (2018).
Li, L. et al. Heterogeneity in estimates of the impact of influenza on population mortality: a systematic review. Am. J. Epidemiol. 187, 378–388 (2018).
Kissling, E. et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: results from the 2021-2022 I-MOVE primary care multicentre study. Influenza Other Respir. Viruses 17, e13069 (2022).
McLean, H. Q. et al. Interim estimates of 2022-23 seasonal influenza vaccine effectiveness - Wisconsin, October 2022-February 2023. MMWR Morb. Mortal. Wkly Rep. 72, 201–205 (2023).
Skowronski, D. M. et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Eur. Surveill. 28, 2300043 (2023).
CDC. Manual for the Surveillance of Vaccine-preventable Disease. Chapter 6, Influenza. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt06-influenza.html (2023).
Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Coffman, R. L., Sher, A. & Seder, R. A. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010).
Geckin, B., Fohse, F. K., Dominguez-Andres, J. & Netea, M. G. Trained immunity: implications for vaccination. Curr. Opin. Immunol. 77, 102190 (2022).
Parker, L. et al. Effects of egg-adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J. Gen. Virol. 97, 1333–1344 (2016).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011). Description of an antibody that bound to a conserved epitope in the fusion subdomain of haemagglutinin and offered protection in mice against influenza A (H1N1) and influenza A (H3N2) viruses and in ferrets against influenza A (H5N1) virus.
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009).
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011).
Kallewaard, N. L. et al. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166, 596–608 (2016).
Xiao, H. et al. Light chain modulates heavy chain conformation to change protection profile of monoclonal antibodies against influenza A viruses. Cell Discov. 5, 21 (2019).
Krammer, F., Li, L. & Wilson, P. C. Emerging from the shadow of hemagglutinin: neuraminidase is an important target for influenza vaccination. Cell Host Microbe 26, 712–713 (2019).
Doyle, T. M. et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res. 100, 567–574 (2013).
Rijal, P. et al. Broadly inhibiting antineuraminidase monoclonal antibodies induced by trivalent influenza vaccine and H7N9 infection in humans. J. Virol. 94, e01182-19 (2020).
Moore, K. A. et al. A research and development (R&D) roadmap for influenza vaccines: looking toward the future. Vaccine 39, 6573–6584 (2021). This describes a pathway to improved and universal influenza vaccines with links to an up-to-date tracking of their research and development.
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021).
Folschweiller, N. et al. Reactogenicity, safety, and immunogenicity of chimeric haemagglutinin influenza split-virion vaccines, adjuvanted with AS01 or AS03 or non-adjuvanted: a phase 1-2 randomised controlled trial. Lancet Infect. Dis. 22, 1062–1075 (2022).
Lewis, N. M. et al. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin. Infect. Dis. 75, 170–175 (2022).
Izurieta, H. S. et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017-2018. J. Infect. Dis. 220, 1255–1264 (2019).
Izurieta, H. S. et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J. Infect. Dis. 222, 278–287 (2020).
Izurieta, H. S. et al. Comparative effectiveness of influenza vaccines among US medicare beneficiaries ages 65 years and older during the 2019-2020 season. Clin. Infect. Dis. 73, e4251–e4259 (2021). The third of a series of reports on the relative vaccine effectiveness of enhanced seasonal influenza vaccines compared with standard egg-based vaccines in preventing hospital encounters among people aged >65 during the last influenza season prior to the emergence of COVID-19.
Boikos, C. et al. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in US adults ≥65 years during the 2017-2018 and 2018-2019 influenza seasons. Clin. Infect. Dis. 73, 816–823 (2021).
Boikos, C. et al. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018-2019 influenza season in the United States. Clin. Infect. Dis. 73, e692–e698 (2021).
Whitney, C. G. et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348, 1737–1746 (2003).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Rachlin, A. et al. Progress toward polio eradication — worldwide, January 2020-April 2022. MMWR Morb. Mortal. Wkly Rep. 71, 650–655 (2022).
de Quadros, C. A., Andrus, J. K., Danovaro-Holliday, M. C. & Castillo-Solorzano, C. Feasibility of global measles eradication after interruption of transmission in the Americas. Expert Rev. Vaccines 7, 355–362 (2008).
Minta, A. A. et al. Progress toward regional measles elimination — worldwide, 2000-2021. MMWR Morb. Mortal. Wkly Rep. 71, 1489–1495 (2022).
Flannery, B. et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018-2019 season. J. Infect. Dis. 221, 8–15 (2020).
Francis, T. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 104, 572–578 (1960). A perspective of the early work done by Francis, Davenport and Hennessey on how the recognition of influenza viruses is affected by an imprint established by the original virus infection governing later antibody responses.
Shanks, G. D., Hussell, T. & Brundage, J. F. Epidemiological isolation causing variable mortality in Island populations during the 1918-1920 influenza pandemic. Influenza Other Respir. Viruses 6, 417–423 (2012).
Mantle, J. & Tyrrell, D. A. An epidemic of influenza on Tristan da Cunha. J. Hyg. 71, 89–95 (1973).
Nogales, A., Martinez-Sobrido, L., Topham, D. J. & DeDiego, M. L. Modulation of innate immune responses by the influenza A NS1 and PA-X proteins. Viruses 10, 708 (2018).
Harper, D. M. et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364, 1757–1765 (2004).
Villa, L. L. et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24, 5571–5583 (2006).
Falsey, A. R. et al. Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N. Engl. J. Med. 388, 609–620 (2023).
Leroux-Roels, I. et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase I/II randomized clinical trial. J. Infect. Dis. 227, 761–772 (2023).
Tsang, T. K. et al. Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study. BMC Med. 20, 230 (2022).
Phillips, R. E. et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354, 453–459 (1991).
GeurtsvanKessel, C. H. et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 7, eabo2202 (2022).
Deng, N., Weaver, J. M. & Mosmann, T. R. Cytokine diversity in the Th1-dominated human anti-influenza response caused by variable cytokine expression by Th1 cells, and a minor population of uncommitted IL-2+IFNγ– Thpp cells. PLoS One 9, e95986 (2014).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Nguyen, T. H. O. et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat. Commun. 12, 2691 (2021).
Herati, R. S. et al. Vaccine-induced ICOS+CD38+ circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways. Cell Rep. Med. 2, 100262 (2021).
Koutsakos, M. et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 10, eaan8405 (2018).
Laidlaw, B. J., Craft, J. E. & Kaech, S. M. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat. Rev. Immunol. 16, 102–111 (2016).
Cullen, J. G. et al. CD4+ T help promotes influenza virus-specific CD8+ T cell memory by limiting metabolic dysfunction. Proc. Natl Acad. Sci. USA 116, 4481–4488 (2019).
Lu, Y. J. et al. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 36, 109696 (2021).
Son, Y. M. et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 6, eabb6852 (2021).
Busselaar, J., Tian, S., van Eenennaam, H. & Borst, J. Helpless priming sends CD8+ T cells on the road to exhaustion. Front. Immunol. 11, 592569 (2020).
Sant, A. J. & McMichael, A. Revealing the role of CD4+ T cells in viral immunity. J. Exp. Med. 209, 1391–1395 (2012).
Cenerenti, M., Saillard, M., Romero, P. & Jandus, C. The era of cytotoxic CD4 T cells. Front. Immunol. 13, 867189 (2022).
Wilkinson, T. M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Brown, D. M., Lampe, A. T. & Workman, A. M. The differentiation and protective function of cytolytic CD4 T cells in influenza infection. Front. Immunol. 7, 93 (2016).
Hua, L. et al. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J. Virol. 87, 11884–11893 (2013).
van Leeuwen, E. M., Remmerswaal, E. B., Heemskerk, M. H., ten Berge, I. J. & van Lier, R. A. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood 108, 3121–3127 (2006).
Chen, M. & Wang, J. Programmed cell death of dendritic cells in immune regulation. Immunol. Rev. 236, 11–27 (2010).
Zweerink, H. J., Courtneidge, S. A., Skehel, J. J., Crumpton, M. J. & Askonas, B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature 267, 354–356 (1977).
Doherty, P. C., Biddison, W. E., Bennink, J. R. & Knowles, B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J. Exp. Med. 148, 534–543 (1978).
Effros, R. B., Doherty, P. C., Gerhard, W. & Bennink, J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 145, 557–568 (1977).
McMichael, A. J., Ting, A., Zweerink, H. J. & Askonas, B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature 270, 524–526 (1977).
Bennink, J. R., Yewdell, J. W. & Gerhard, W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature 296, 75–76 (1982).
Gotch, F., McMichael, A., Smith, G. & Moss, B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 165, 408–416 (1987).
Townsend, A. R., Skehel, J. J., Taylor, P. M. & Palese, P. Recognition of influenza A virus nucleoprotein by an H-2-restricted cytotoxic T-cell clone. Virology 133, 456–459 (1984).
Topham, D. J. & Reilly, E. C. Tissue-resident memory CD8+ T cells: from phenotype to function. Front. Immunol. 9, 515 (2018).
Farber, D. L. Tissues, not blood, are where immune cells function. Nature 593, 506–509 (2021).
Zheng, M. Z. M. & Wakim, L. M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15, 379–388 (2022).
Rotrosen, E. & Kupper, T. S. Assessing the generation of tissue resident memory T cells by vaccines. Nat. Rev. Immunol. 23, 655–665 (2023).
Ray, S. J. et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179 (2004). This study of lung-resident T cells showed that CD49A was required for maintenance in the lungs but not lymphoid tissues, and loss of the lung-resident population after CD49A blockade was associated with reduced resistance to subsequent influenza virus re-infection.
Wu, T. et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2014).
Ariotti, S. et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl Acad. Sci. USA 109, 19739–19744 (2012).
Pizzolla, A. et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 128, 721–733 (2018).
Gaide, O. et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 21, 647–653 (2015).
Park, S. L. et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19, 183–191 (2018).
Ely, K. H., Cookenham, T., Roberts, A. D. & Woodland, D. L. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 176, 537–543 (2006).
Slutter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017).
Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 4, eaav5581 (2019).
Moyron-Quiroz, J. E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Takamura, S. Niches for the long-term maintenance of tissue-resident memory T cells. Front. Immunol. 9, 1214 (2018).
Yap, K. L., Ada, G. L. & McKenzie, I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273, 238–239 (1978).
Taylor, P. M. & Askonas, B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology 58, 417–420 (1986).
Graham, M. B., Braciale, V. L. & Braciale, T. J. Influenza virus-specific CD4+ T helper type 2T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 180, 1273–1282 (1994).
McKinstry, K. K. et al. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 122, 2847–2856 (2012).
Scherle, P. A., Palladino, G. & Gerhard, W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148, 212–217 (1992).
McMichael, A. J., Gotch, F. M., Noble, G. R. & Beare, P. A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309, 13–17 (1983).
Paterson, S. et al. Innate-like gene expression of lung-resident memory CD8+ T cells during experimental human influenza: a clinical study. Am. J. Respir. Crit. Care Med. 204, 826–841 (2021). This human influenza virus challenge study also incorporated local sampling by bronchoalveolar lavage, providing valuable information on T cell responses in the lung.
Omokanye, A. et al. Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity. Mucosal Immunol. 15, 717–729 (2022).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Hayward, A. C. et al. Natural T cell-mediated protection against seasonal and pandemic influenza. Results of the flu watch cohort study. Am. J. Respir. Crit. Care Med. 191, 1422–1431 (2015).
Zhao, M. et al. Prolonged evolution of virus-specific memory T cell immunity after severe avian influenza A (H7N9) virus infection. J. Virol. 92, e01024–18 (2018).
Von Holle, T. A. & Moody, M. A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 10, 1457 (2019).
Jegaskanda, S. et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J. Infect. Dis. 214, 945–952 (2016).
Edgar, J. E. et al. Antibodies elicited in humans upon chimeric hemagglutinin-based influenza virus vaccination confer FcγR-dependent protection in vivo. Proc. Natl Acad. Sci. USA 120, e2314905120 (2023).
Laidlaw, B. J. et al. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 9, e1003207 (2013).
Paluch, C., Santos, A. M., Anzilotti, C., Cornall, R. J. & Davis, S. J. Immune checkpoints as therapeutic targets in autoimmunity. Front. Immunol. 9, 2306 (2018).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 (2009).
Gray, G. E. et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 384, 1089–1100 (2021).
Allan, W. H., Madeley, C. R. & Kendal, A. P. Studies with avian influenza A viruses: cross protection experiments in chickens. J. Gen. Virol. 12, 79–84 (1971).
Beutner, K. R. et al. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J. Infect. Dis. 140, 844–850 (1979).
Couch, R. B., Kasel, J. A., Gerin, J. L., Schulman, J. L. & Kilbourne, E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 129, 411–420 (1974).
Skehel, J. J. Polypeptide synthesis in influenza virus-infected cells. Virology 49, 23–36 (1972).
Shapiro, G. I., Gurney, T. Jr & Krug, R. M. Influenza virus gene expression: control mechanisms at early and late times of infection and nuclear-cytoplasmic transport of virus-specific RNAs. J. Virol. 61, 764–773 (1987).
Hu, Y., Sneyd, H., Dekant, R. & Wang, J. Influenza A virus nucleoprotein: a highly conserved multi-functional viral protein as a hot antiviral drug target. Curr. Top. Med. Chem. 17, 2271–2285 (2017).
Babar, M. M. & Zaidi, N. U. Protein sequence conservation and stable molecular evolution reveals influenza virus nucleoprotein as a universal druggable target. Infect. Genet. Evol. 34, 200–210 (2015).
Wraith, D. C., Vessey, A. E. & Askonas, B. A. Purified influenza virus nucleoprotein protects mice from lethal infection. J. Gen. Virol. 68, 433–440 (1987).
Andrew, M. E., Coupar, B. E., Boyle, D. B. & Ada, G. L. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand. J. Immunol. 25, 21–28 (1987).
Christensen, J. P., Doherty, P. C., Branum, K. C. & Riberdy, J. M. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8+ T-cell memory. J. Virol. 74, 11690–11696 (2000).
Tite, J. P. et al. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology 71, 202–207 (1990).
Ulmer, J. B. et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259, 1745–1749 (1993).
Ulmer, J. B. et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72, 5648–5653 (1998).
Xie, C., Yao, R. & Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 8, 162 (2023).
Hochheiser, K. et al. Cutting edge: the RIG-I ligand 3pRNA potently improves CTL cross-priming and facilitates antiviral vaccination. J. Immunol. 196, 2439–2443 (2016).
Valencia-Hernandez, A. M. et al. Complexing CpG adjuvants with cationic liposomes enhances vaccine-induced formation of liver TRM cells. Vaccine 41, 1094–1107 (2023).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Iborra, S. et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity 45, 847–860 (2016).
Wakim, L. M., Smith, J., Caminschi, I., Lahoud, M. H. & Villadangos, J. A. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 8, 1060–1071 (2015).
Vatzia, E. et al. Immunization with matrix-, nucleoprotein and neuraminidase protects against H3N2 influenza challenge in pH1N1 pre-exposed pigs. NPJ Vaccines 8, 19 (2023).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Pan, Y. et al. Epicutaneous immunization with modified vaccinia Ankara viral vectors generates superior T cell immunity against a respiratory viral challenge. Vaccines 6, 1 (2021).
Oberhardt, V. et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 597, 268–273 (2021).
Gao, F. et al. Spheromers reveal robust T cell responses to the Pfizer/BioNTech vaccine and attenuated peripheral CD8+ T cell responses post SARS-CoV-2 infection. Immunity 56, 864–878.e4 (2023).
Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 374, eabj9853 (2021).
Ssemaganda, A. et al. Expansion of cytotoxic tissue-resident CD8+ T cells and CCR6+CD161+CD4+ T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat. Commun. 13, 3357 (2022).
Mitsi, E. et al. Respiratory mucosal immune memory to SARS-CoV-2 after infection and vaccination. Nat. Commun. 14, 6815 (2023).
Xiong, F. et al. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 12, 2256422 (2023).
Lee, I. T. et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis. Nat. Commun. 14, 3631 (2023).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25, 1316–1327 (2017).
Feldman, R. A. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37, 3326–3334 (2019).
Ewer, K. J. et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 27, 270–278 (2021).
Stephenson, K. E. et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 325, 1535–1544 (2021).
Knight, F. C. & Wilson, J. T. Engineering vaccines for tissue-resident memory T cells. Adv. Ther. 4, 2000230 (2021).
Lillie, P. J. et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 55, 19–25 (2012).
Evans, T. G. et al. Efficacy and safety of a universal influenza A vaccine (MVA-NP+M1) in adults when given after seasonal quadrivalent influenza vaccine immunisation (FLU009): a phase 2b, randomised, double-blind trial. Lancet Infect. Dis. 22, 857–866 (2022).
Del Campo, J. et al. OVX836 heptameric nucleoprotein vaccine generates lung tissue-resident memory CD8+ T-cells for cross-protection against influenza. Front. Immunol. 12, 678483 (2021).
Leroux-Roels, I. et al. Immunogenicity, safety, and preliminary efficacy evaluation of OVX836, a nucleoprotein-based universal influenza A vaccine candidate: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Infect. Dis. 22, 857–866 (2023).
Leroux-Roels, I. et al. Randomized, double-blind, reference-controlled, phase 2a study evaluating the immunogenicity and safety of OVX836, a nucleoprotein-based influenza vaccine. Front. Immunol. 13, 852904 (2022).
Pleguezuelos, O. et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. Vaccines 5, 22 (2020).
Taylor, P. M., Esquivel, F. & Askonas, B. A. Murine CD4+ T cell clones vary in function in vitro and in influenza infection in vivo. Int. Immunol. 2, 323–328 (1990).
Enelow, R. I. et al. Structural and functional consequences of alveolar cell recognition by CD8+ T lymphocytes in experimental lung disease. J. Clin. Invest. 102, 1653–1661 (1998).
Small, B. A. et al. CD8+ T cell-mediated injury in vivo progresses in the absence of effector T cells. J. Exp. Med. 194, 1835–1846 (2001).
Hoskins, T. W., Davies, J. R., Smith, A. J., Miller, C. L. & Allchin, A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1, 33–35 (1979).
Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 23, 186–193 (2022).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563 (2017).
Zens, K. D., Chen, J. K. & Farber, D. L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, e85832 (2016).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 7, eadd4853 (2022).
Brenna, E. et al. CD4+ T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-Tfh CD4+ cells. Cell Rep. 30, 137–152.e5 (2020).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021).
Valkenburg, S. A. & Poon, L. L. M. Universal influenza vaccines are futile when benchmarked against seasonal influenza vaccines. Lancet Infect. Dis. 22, 750–751 (2022).
Wu, N. et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 11, 439–452 (2023).
Zheng, X. et al. Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 15, e1008036 (2019).
Gouglas, D. et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Health 6, e1386–e1396 (2018).
Bloom, D. E., Cadarette, D. & Tortorice, D. L. An ounce of prevention: our approach to vaccine finance is ill-suited to addressing epidemic risk. Finance Dev. 57, 54–57 (2020).
Plotkin, S., Robinson, J. M., Cunningham, G., Iqbal, R. & Larsen, S. The complexity and cost of vaccine manufacturing — an overview. Vaccine 35, 4064–4071 (2017).
Acknowledgements
J.W.McC. is supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC001030), the Medical Research Council (FC001030) and the Wellcome Trust (FC001030). A.J.McM. is supported by the CAMS-Oxford Institute.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
T.R.M. is a member of the Osivax scientific advisory board. A.J.McM. is a member of the scientific advisory boards of Osivax, Oxford Vacmedix and T-Cypher Bio. A.L.V. is an employee and shareholder of Osivax. J.W.McC. has provided advice to CSL Seqirus, Sanofi Pasteur, Pfizer and Iceni Diagnostics. J.W.A. has provided scientific advice to Osivax, IosBios Ltd., Mynvax and Blue Lake Biotechnology. He is also a shareholder of Sanofi, Vaxcyte, Moderna and OVO Biomanufacturing Ltd.
Peer review
Peer review information
Nature Reviews Immunology thanks Katherine Kedzierska and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CDC. About Diphtheria, Tetanus, and Pertussis Vaccines: https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html
CDC. About HPV Vaccines: https://www.cdc.gov/vaccines/vpd/hpv/hcp/vaccines.html
CDC. Past Seasons Estimated Influenza Disease Burden: https://www.cdc.gov/flu/about/burden/past-seasons.html
Influenza Vaccines Roadmap: https://ivr.cidrap.umn.edu/universal-influenza-vaccine-technology-landscape
Measles & Rubella Partnership: https://measlesrubellainitiative.org/measles-rubella-strategic-framework-2021-2030/
Polio Global Eradication Initiative: https://polioeradication.org/
WHO. FluNet Summary: https://www.who.int/tools/flunet/flunet-summary
WHO. Measles: https://www.who.int/news-room/fact-sheets/detail/measles
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mosmann, T.R., McMichael, A.J., LeVert, A. et al. Opportunities and challenges for T cell-based influenza vaccines. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01030-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01030-8