Abstract
The radiation-induced bystander effect (RIBE) refers to a unique process in which factors released by irradiated cells or tissues exert effects on other parts of the animal not exposed to radiation, causing genomic instability, stress responses and altered apoptosis or cell proliferation1,2,3. Although RIBEs have important implications for radioprotection, radiation safety and radiotherapy, the molecular identities of RIBE factors and their mechanisms of action remain poorly understood. Here we use Caenorhabditis elegans as a model in which to study RIBEs, and identify the cysteine protease CPR-4, a homologue of human cathepsin B, as the first RIBE factor in nematodes, to our knowledge. CPR-4 is secreted from animals irradiated with ultraviolet or ionizing gamma rays, and is the major factor in the conditioned medium that leads to the inhibition of cell death and increased embryonic lethality in unirradiated animals. Moreover, CPR-4 causes these effects and stress responses at unexposed sites distal to the irradiated tissue. The activity of CPR-4 is regulated by the p53 homologue CEP-1 in response to radiation, and CPR-4 seems to exert RIBEs by acting through the insulin-like growth factor receptor DAF-2. Our study provides crucial insights into RIBEs, and will facilitate the identification of additional RIBE factors and their mechanisms of action.
Similar content being viewed by others
Main
We tested whether C. elegans can serve as an animal model to study RIBE using ultraviolet (UV) radiation, because UV-induced damage in C. elegans is well characterized4. Wild-type (N2) animals cultured in liquid S-medium were irradiated with 100 J m−2 UV or sham-irradiated. This UV dosage induced significant embryonic lethality (Extended Data Fig. 1a), which was exacerbated in cep-1(gk138) animals defective in the C. elegans p53 homologue CEP-1 that is involved in DNA damage repair4,5,6. The medium used to culture irradiated and sham-irradiated animals was defined as ‘UV conditioned medium’ (UV-CM) and ‘UV control’ (UV-ctrl), respectively, and used to treat unexposed animals (Fig. 1a). N2 animals treated with UV-CM showed increased embryonic lethality compared with those treated with UV-ctrl (Extended Data Fig. 1b), indicating that UV-CM contains substances capable of inducing damage in unexposed animals. UV-CM also reduced germ-cell death in ced-1(e1735) animals, which have many unengulfed apoptotic cells that sensitize the detection of apoptosis, in a manner dependent on the UV dosage (Fig. 1b), reaching maximal death inhibitory activity at 100 J m−2. These results are consistent with reports that reduced apoptosis or increased survival of unexposed cells is one of the endpoints of RIBEs3,7,8,9.
a, Schematic presentation of the RIBE assay in C. elegans (Methods). b, c, ced-1(e1735) L4 larvae were cultured in UV-CM from N2 animals irradiated at the indicated dosage (b) or UV-CM (100 J m−2) treated with trypsin protease (50 ng μl−1) (c). Germ-cell corpses were scored after 48 h. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. NS, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, two-sided, unpaired t-test. d, e, Mass spectrometry analysis. Concentrated >10 kDa UV-CM and UV-ctrl fractions were resolved by SDS–PAGE and silver stained (d). Protein identities in bands unique to UV-CM (marked by numbers) are shown (e). f, CPR-4::Flag was secreted into UV-CM from Pcpr-4::cpr-4::flag animals. UV-CM and UV-ctrl (1 μg μl−1) were resolved by SDS–PAGE and detected by immunoblotting (IB). For gel source data, see Supplementary Fig. 1.
We probed the nature of RIBE factors by treating UV-CM with enzymes that destroy DNA, RNA or proteins. The apoptosis-inhibitory activity in UV-CM was resistant to the treatment of DNase or RNase (Extended Data Fig. 2a, b), but obliterated by the trypsin protease (Fig. 1c), suggesting that the RIBE factors are proteins. UV-CM collected from cell-death-defective ced-3(n2433) animals, germline-deficient glp-1(e2141) animals, or N2 animals fed with dead bacteria retained the death inhibitory activity (Extended Data Fig. 2c–f), indicating that the RIBE factors are unlikely to be generated by bacteria or by-products of cell death induced by radiation, and can be produced without the germ line.
Using 10-kDa molecular mass cut-off filter units, we separated UV-CM into two fractions, one containing proteins likely to be larger than 10 kDa, and one with proteins smaller than 10 kDa. The RIBE activity appeared in the greater than 10 kDa fraction (Extended Data Fig. 3a), which was resolved on a SDS–polyacrylamide gel (Fig. 1d). Protein bands unique to UV-CM were analysed by mass spectrometry, from which 19 proteins were identified (Fig. 1e, Extended Data Fig. 4 and Extended Data Table 1).
We used RNA interference (RNAi) to examine whether 1 of the 19 genes is responsible for RIBE. UV-CM from cpr-4 RNAi-treated animals displayed a greatly reduced RIBE activity, whereas UV-CM from animals treated with RNAi of other genes retained the RIBE activity (Extended Data Fig. 3b). cpr-4 encodes a homologue of the mammalian cathepsin B lysosomal protease, which is secreted to act as an extracellular protease10,11,12. Because a deletion mutation (tm3718) in cpr-4, which removes one-third of the CPR-4 protein (Extended Data Fig. 5a), obliterated the RIBE activity and a single-copy integrated transgene carrying a cpr-4 genomic fragment with a carboxyl terminal Flag tag (Pcpr-4::cpr-4::flag) restored RIBE to cpr-4(tm3718) animals (Fig. 2a), cpr-4 is required for this RIBE activity in UV-CM.
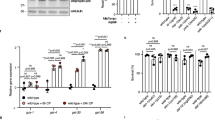
a, d, e, Conditioned medium (0.1 μg μl−1) from the indicated strains (a, e) or 2.8 μM of recombinant tCPR-4 proteins (d) were used to treat ced-1(e1735) animals as in Fig. 1b. b, c, f, Protease activity of conditioned medium (0.1 μg μl−1) from the indicated strains (b, f) or 2.8 μM tCPR-4 proteins (c). Immunoblotting image of tCPR-4 proteins is below c. RFU, relative fluorescent units. g, CPR-4::Flag was secreted into IR-CM from Pcpr-4::cpr-4::flag animals. IR-CM and IR-ctrl (1 μg μl−1) resolved by SDS–PAGE were detected by immunoblotting. h, Relative cpr-4 mRNA levels (fold change) in the indicated strains were determined by qRT–PCR, compared to those of sham-irradiated samples (control). Data are mean ± s.e.m. (a–f, h). The numbers of gonad arms scored are indicated inside the bars (a, d, e), and n = 6 in each group for other assays (b, c, f, h). ***P < 0.001, two-sided, unpaired t-test. For gel source data, see Supplementary Fig. 1.
We examined whether CPR-4 is secreted into the medium upon UV irradiation. CPR-4::Flag was detected in UV-CM, but not in UV-ctrl, from Pcpr-4::cpr-4::flag animals (Fig. 1f). Immunodepletion of CPR-4::Flag from UV-CM of Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals abolished its RIBE activity (Extended Data Fig. 2g, h), confirming that secreted CPR-4 is the RIBE factor in UV-CM. Because UV-CM from cep-1(gk138) animals lost the RIBE activity (Fig. 2a) and UV-CM from cep-1(gk138); Pcpr-4::cpr-4::flag animals showed greatly reduced secretion of CPR-4::Flag (Extended Data Fig. 3c), the CPR-4-mediated RIBEs are induced through a cep-1-dependent mechanism, like some reported p53-dependent RIBEs in mammals3,13.
RIBEs often refer to intra-animal bystander effects. We tested whether localized UV irradiation (LUI) at the head of an animal might induce bystander effects in other areas of the animal not exposed to the radiation (Fig. 3a). Using a stress-response reporter, Phsp-4::gfp (zcIs4), that also reacts to radiation14,15, we observed increased green fluorescent protein (GFP) expression in multiple unexposed regions of LUI-treated zcIs4 animals 24 h after radiation, including strong GFP expression in the posterior region (Fig. 3b, c). This bystander response was strongest in L4 larvae (Fig. 3d), but lost in cpr-4(tm3718) and cep-1(gk138) mutants (Fig. 3e; Extended Data Fig. 6a), indicating that both cpr-4 and cep-1 are required for intra-animal RIBE. LUI also led to increased embryonic lethality in unexposed progeny (Fig. 3a, f) and reduced germ-cell death in non-irradiated posterior gonads in a cpr-4-dependent manner (Fig. 3a, g), indicating that LUI-induced intra-animal RIBEs are similar to inter-animal RIBEs induced by UV-CM, and that CPR-4 is a bona fide RIBE factor.
a, Schematic presentation of an intra-animal model to assay RIBE. The pharyngeal area of the animal was irradiated and RIBEs were analysed in three unexposed areas as indicated (Methods). b, c, Representative images (at least 20) of Phsp-4::gfp animals with (UV) or without (UV-control) LUI. Animal tails to the top right. Scale bars, 50 μm. d, Assays of the Phsp-4::gfp response to LUI at different developmental stages. e–g, The indicated strains were analysed for the Phsp-4::gfp response (e), F1 embryonic lethality (f), and germ-cell corpses in posterior gonads (g) 24 h after LUI. Some experiments in e and all in f were done at 25 °C. Data are mean ± s.e.m. The numbers of animals (d, e), plates with embryos (f), or gonad arms (g) scored are indicated inside the bars. Total numbers of embryos scored: 928, 1,460, 769, 761, 522 and 537, from left to right (f). *P < 0.05, **P < 0.01, ***P < 0.001, two-sided, unpaired t-test.
CPR-4 and cathepsin B are highly conserved and have identical catalytic residues (Extended Data Fig. 5b), including the active-site cysteine and a histidine residue acting as a general base16. Using a cathepsin B-specific fluorogenic substrate, z-Arg-Arg-AMC, we detected cathepsin B-like protease activity in UV-CM, but not in UV-ctrl, from N2 animals (Fig. 2b). This activity was absent in UV-CM from cpr-4(tm3718) animals, greatly reduced in UV-CM from cep-1(gk138) animals, but restored in Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals, confirming the hypothesis that CPR-4 confers this cathepsin B-like activity in UV-CM through a cep-1-dependent mechanism.
We tested whether recombinant CPR-4 recapitulated the RIBE activity. A truncated CPR-4 lacking its signal peptide (residues 1–15), tCPR-4, exhibited a similar protease activity to that of recombinant human cathepsin B (rhCTSB) (Extended Data Fig. 3d). Mutations altering the conserved catalytic residues, C109A and H281A, abolished the protease activity of tCPR-4 (Fig. 2c), whereas a mutation (N301A) changing a non-catalytic residue did not affect tCPR-4 protease activity16. Like UV-CM from N2 animals, tCPR-4, tCPR-4(N301A), and rhCTSB reduced germ-cell corpses (Fig. 2d; Extended Data Fig. 3e) and increased embryonic lethality (Extended Data Fig. 1c), whereas tCPR-4(H281A) and tCPR-4(C109A) failed to do so, indicating that the CPR-4 protease activity is crucial for its RIBE activities.
We tested conditioned medium from animals irradiated by a different radiation source, ionizing radiation (IR-CM), and its sham-irradiated control (IR-ctrl). IR-CM from N2 or Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals reduced germ-cell corpses in ced-1(e1735) animals, whereas IR-CM from cpr-4(tm3718) or cep-1(gk138) animals had no such activity (Fig. 2e). Similarly, IR-CM, but not IR-ctrl, from N2 animals caused increased embryonic lethality (Extended Data Fig. 1b) and contained a cathepsin B-like activity that was lost in IR-CM from cpr-4(tm3718) or cep-1(gk138) animals, but restored in Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals (Fig. 2f). Moreover, secreted CPR-4::Flag was detected in IR-CM, but not in IR-ctrl, from Pcpr-4::cpr-4::flag animals (Fig. 2g). Therefore, CPR-4 is a shared RIBE factor induced by different radiation sources.
Using quantitative reverse transcription PCR (qRT–PCR) analysis, we found that the transcription of the cpr-4 gene in N2 animals was increased by approximately 1.6-fold after UV or IR irradiation, compared with sham-irradiated controls (Fig. 2h). By contrast, cpr-4 transcription in cep-1(gk138) animals was not altered by either radiation. These results indicate that ionizing and non-ionizing radiation increases cpr-4 transcription through a CEP-1-dependent mechanism, leading to the synthesis of more CPR-4 proteins and enhanced secretion of CPR-4.
Using a single-copy insertion transgene carrying a cpr-4 transcriptional fusion with GFP and a nuclear localization signal (Pcpr-4::nls::gfp), we examined when and where cpr-4 is expressed. In N2 animals, NLS::GFP expression was not detected in embryos, was observed in the intestine of early stage larvae (L1–L3), peaked at the L4 larval stage, and declined when animals entered adulthood (Extended Data Fig. 7a–h, j). Similar spatiotemporal NLS::GFP expression patterns were observed in cep-1(gk138); Pcpr-4::nls::gfp animals (Extended Data Fig. 7i, j). When irradiated with UV, N2 animals, but not cep-1(gk138) animals carrying Pcpr-4::nls::gfp, showed increased NLS::GFP expression (Extended Data Fig. 7k), confirming that radiation induces increased cpr-4 transcription through a cep-1-dependent mechanism.
To investigate the effects of secreted CPR-4 in vivo, we generated transgenic Pmyo-2::CPR-4::mCherry animals expressing mCherry-tagged CPR-4 specifically in C. elegans pharynx under the control of the myo-2 gene promoter (Extended Data Fig. 8a). As expected of a secreted protein, CPR-4::mCherry was made in and secreted from the pharynx and taken up by cells in the whole body, including the phagocytic coelomocytes (Extended Data Fig. 8a, arrowheads). Removal of the CPR-4 signal peptide blocked tCPR-4::mCherry secretion from the pharynx in transgenic animals (Extended Data Fig. 8b). Like UV-CM, IR-CM or LUI treatment, pharyngeal expression of CPR-4::mCherry increased embryonic lethality, decreased germ-cell death, and in addition, caused substantial larval arrest (Extended Data Fig. 8c, d), which were not seen or greatly attenuated in animals expressing tCPR-4::mCherry or catalytically inactive CPR-4::mCherry proteins. These results from ectopic expression of CPR-4 provide further evidence to support a long-range signalling role of CPR-4 as a RIBE factor.
Given the various RIBEs mediated by CPR-4, we investigated how CPR-4 influences unexposed cells or animals by examining genes that affect multiple cellular processes. The daf-2 gene, which encodes a C. elegans orthologue of the human insulin/IGF receptor and regulates several signalling pathways17,18,19,20, was examined, as reduced daf-2 activity increases lifespan and stress resistance17,18 and decreases germ, muscle and neuronal cell death induced by genotoxic and hypoxic stresses19,20. Similarly, reduced daf-2 function by a temperature-sensitive mutation (e1370) decreased physiological germ-cell death (Fig. 4a). Notably, purified tCPR-4 did not further reduce germ-cell death in the ced-1(e1735); daf-2(e1370) mutant (Fig. 4a), suggesting that tCPR-4 and daf-2 act in the same pathway to affect germ-cell death. Moreover, tCPR-4 did not reduce germ-cell death in ced-1(e1735); pdk-1(sa680) animals, which are defective in the PDK-1 kinase, a key downstream signalling component of DAF-221, but could do so in daf-16(mu86) ced-1(e1735) animals, which lack DAF-1622,23, one of the major transcription factors acting downstream of DAF-2 (Extended Data Fig. 6b). We observed similar results using the LUI assays in which inactivation of daf-2 and pdk-1, but not daf-16, prevented increased GFP expression from Phsp-4::gfp in the posterior unexposed regions (Fig. 3e and Extended Data Fig. 6a) and loss of daf-2 blocked increased embryonic lethality and reduced germ-cell death in unexposed tissues (Fig. 3f, g). Because loss of daf-2 did not seem to affect the secretion of CPR-4 into UV-CM or the apoptosis-inhibitory activity of UV-CM (Extended Data Fig. 6c, d), these results support a model in which the secreted CPR-4 acts through the DAF-2 insulin/IGF receptor and the PDK-1 kinase, but not the DAF-16 transcription factor, to exert RIBEs in unexposed cells.
a, b, L4 larvae of the indicated strains were treated with 2.8 μM tCPR-4 or buffer control for 48 h. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. **P < 0.01, ***P < 0.001, two-sided, unpaired t-test.
Because daf-2 also affects germ-cell proliferation24,25, we examined whether tCPR-4 treatment alters germ-cell proliferation by scoring the number of nuclei in the germline mitotic region24. tCPR-4 treatment of N2 animals resulted in more germ-cell nuclei and more metaphase nuclei in the mitotic zone (Fig. 4b), suggesting a stimulating effect. Reduced daf-2 activity or loss of cep-1 blocked increased germ-cell proliferation induced by tCPR-4 (Fig. 4b and Extended Data Fig. 6e), indicating that tCPR-4 promotes germ-cell proliferation through DAF-2 and CEP-1.
RIBEs are a major factor in determining the efficacy and success of radiotherapy in cancer treatment2,26,27, not only because they affect and cause damage in non-irradiated cells, but also because they can affect irradiated cells through paracrine signalling. Thus, identification of RIBE factors is a fundamental issue in cancer radiotherapy and radioprotection1. Using the C. elegans animal model, we identify the cathepsin B homologue CPR-4 as the major RIBE factor that induces several, typical RIBEs1,27, including apoptosis inhibition and increased cell proliferation, lethality and stress response. In mammals, cathepsin B is secreted from lysosomes to exert extracellular activities, including regulation of apoptosis, and has roles in neoplastic and inflammatory disease states28,29. Recent studies show that extracellular cathepsin B enhances breast cancer-resistance to drug-induced apoptosis during chemotherapy30, which is consistent with our observations in C. elegans. We show that radiation increases cpr-4 transcription and CPR-4 protein production and secretion in C. elegans through a p53/CEP-1-dependent mechanism. The secreted CPR-4 then induces multiple RIBE responses, either directly or indirectly, by regulating the activity of the DAF-2 insulin/IGF receptor that is crucial for several conserved signalling pathways, from ageing, stress response and metabolism to apoptosis17,18. Therefore, our study provides crucial insights into the elusive RIBE phenomenon, not only on how it is generated and what the RIBE factor is, but also on how the RIBE factor impacts non-irradiated cells. This C. elegans model will facilitate the identification of additional RIBE factors and underlying mechanisms in worms and other organisms.
Methods
No statistical methods were used to predetermine sample size.
Strains and culture conditions
We cultured C. elegans strains at 20 °C using standard procedures31, unless otherwise noticed. We used the N2 Bristol strain as the wild-type strain. The following stains were used in the genetic analyses: LGI, cep-1(gk138), daf-16(mu86), ced-1(e1735); LGII, single copy insertion of Pcpr-4::cpr-4::flag32, single copy insertion of Pcpr-4::nls::gfp; LGIII, daf-2(e1370), glp-1(e2141); LGV, cpr-4(tm3718), zcIs4 (Phsp-4::gfp); LGX, pdk-1(sa680). Each single-copy insertion transgene was backcrossed at least four times with N2 animals before being used.
Irradiation
Adult animals grown on Nematode Growth Media (NGM) plates or in plastic tubes with liquid culture media were irradiated at room temperature using a UV-cross-linker or a 60Co radiation source. The dosage of UV irradiation was 100 J m−2. The dosage of 60Co irradiation was 500 Gy at a dosage rate approximately 33.3 Gy min−1. Plates were returned to 20 °C incubators immediately after irradiation. Plastic tubes were placed in a 20 °C shaker after irradiation to generate conditioned medium. Sham-irradiated controls were used in all irradiation experiments.
Generation of conditioned medium from irradiated animals
C. elegans animals close to starving were washed off from three NGM plates (6 cm in diameter) and cultured for 6 days in 250 ml of S-medium (100 mM NaCl, 5.8 mM K2HPO4, 44 mM KH2PO4, 0.013 mM cholesterol, 1 mM citric acid monohydrate, 9 mM tri-potassium citrate monohydrate, 0.05 mM disodium EDTA, 0.025 mM FeSO4, 0.01 mM MnCl2, 0.01 mM ZnSO4, 0.001 mM CuSO4, 1.5 mM CaCl2, 3 mM MgSO4, 0.13 mM ampicillin, 0.007 mM streptomycin sulfate, 0.16 mM neomycin sulfate, and 0.02 mM nystatin) using plentiful Escherichia coli strain HB101 as a food source. The animals were harvested by precipitation at 4 °C for 10 min, which collected mostly adults, and washed with S-medium three times. We adjusted the animal density to approximately 2 animals per microlitre in S-medium, transferred them to a quartz plate (with lid), and irradiated them using UV with the desired dosages or sham-irradiated. For IR irradiation, animals at the same density were transferred to 15-ml Corning centrifuge tubes and irradiated using 500 Gy IR or sham-irradiated. The irradiated or sham-irradiated animals were washed with fresh S-medium, transferred to 15-ml Corning centrifuge tubes in 6-ml S-medium supplemented with the HB101 bacteria, and grown in a 20 °C shaker for 24 h with constant 200 r.p.m. shaking. After that, we removed the animals and bacteria by centrifugation at 1,700g for 10 min and filtrated the medium with a 0.22-μm filter unit to obtain conditioned medium. The conditioned medium was then concentrated by passing through a 10-kDa ultrafiltration tube (Amicon Ultra-15, Millipore) and adjusted to 0.1 μg μl−1 total protein concentration using S-medium. To generate UV-CM and UV-ctrl from Pcpr-4::cpr-4::flag; daf-2(e1370); cpr-4(tm3718) animals, starved plates containing the animals were chunked to 300 new NGM plates, which were placed at 20 °C for 2 days before being shifted to 25 °C for one more day. UV-irradiated or sham-irradiated animals were grown in a 25 °C shaker for 24 h to obtain UV-CM and UV-ctrl.
Localized irradiation in C. elegans
C. elegans L4 larvae were mounted on an agarose pad (2%) with 10 nM sodium azide and irradiated at the head region using a Nikon A1 laser scanning confocal on an inverted Ti-E microscope with a 40 × /0.9 NA Plan Apo Lambda objective lens. At installation, the 405 nm laser power, which is very close to the wavelength of UV, was measured at 23.32 mW at the fibre. Irradiation was performed using 60% 405 nm laser power at 512 × 512 with a pixel size of 0.58 μm × 0.58 μm for 2.2 μs pixel−1. Using a Thor labs power meter (PM100D) and photosensor (S140C), we measured the power at the sample plane to be approximately 0.25–0.30 mW. This corresponds to approximately 0.75–0.89 mW μm-2 at the sample. For sham-irradiation controls, a region slightly away from the animal on the agarose pad was irradiated. After irradiation, the animals were immediately rescued from the agarose pad and transferred to a regular NGM plate to recover at 20 °C for 24 h or at 25 °C for 20 h (embryonic lethality assays) before being assayed for intra-animal bystander effects. Three assays were conducted to monitor intra-animal bystander effects in unexposed areas. They are germ-cell corpse assays in the posterior gonads, embryonic lethality assays of the F1 progeny of irradiated animals, and Phsp-4::gfp stress response assays in the posterior region. For Phsp-4::gfp stress response assays, experiments using the daf-2(e1370ts) strains and corresponding control strains were performed at 25 °C after LUI. For the embryonic lethality assays, after 20 h recovery at 25 °C, irradiated or sham-irradiated animals were placed on NGM plates to lay eggs for 4 h at 25 °C and then transferred to new NGM plates. After two more transfers, the animals were discarded. The number of eggs that did not hatch (scored as dead eggs) and the number of eggs that developed into larvae were scored and used to determine the rate of embryonic lethality.
Formaldehyde-treated bacteria as the food source
HB101 bacteria were treated with 3.7% formaldehyde for 10 min, washed three times with S-medium, and collected by centrifugation. The death of bacteria was verified by spreading them on a plate with no antibiotics and observing no bacterial colony. The bacterial pellets were added to S-medium to grow worms.
RNAi experiments
RNAi experiments were performed using a bacterial feeding protocol33. HT115 bacteria transformed with the pPD129.36-cpr-4 or pPD129.36 plasmid were used in cpr-4 RNAi and control RNAi experiments, respectively. Bacterial clones used in other RNAi experiments are from an RNAi library purchased from ThermoFisher. To perform RNAi experiments in liquid culture, three NGM plates with RNAi bacteria were used to feed 30 larval stage 4 (L4) N2 animals until the plates were almost starved. We then washed the animals off the plates and transferred them to glass flasks with 250 ml of S-medium containing 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and the RNAi bacteria and grew them for one more generation. The procedure to obtain conditioned medium is similar to that described above.
Growing C. elegans animals in 96-well plates
HB101 bacteria were mixed with 100 μl conditioned medium (0.1 μg μl−1) or 100 μl S-medium containing 2.8 μM of recombinant tCPR-4 proteins (wild-type or mutant) or 0.27 μM recombinant human cathepsin B in a 96-well plate. Approximately 60 L4 larvae were transferred into each well of the plate. After being cultured in liquid media for 48 h, these animals were scored for the numbers of germ cell corpses and mitotic nuclei.
Enzyme treatment of conditioned medium
The nature of the RIBE factor was analysed by treating conditioned medium with different enzymes. 1 μl DNase (1 U μl−1, QIAGEN), 1 μl RNase (100 μg μl−1, QIAGEN) or 1 μl trypsin (5 μg μl−1, Sigma) was mixed with 100 μl conditioned medium for 1 h at 30 °C. The treated or untreated conditioned medium was then used to culture ced-1(e1735) animals for 48 h at 20 °C in a 96-well plate.
Quantification of germ-cell corpses
L4 animals were cultured in liquid media in a 96-well plate as described above. After 48 h, they were transferred to NGM plates and allowed to recover for 1 h at 20 °C. The animals were then anaesthetized by 20 mM NaN3, mounted onto 2% agar pad, and scored under Nomarski optics. For transgenic animals expressing CPR-4 in the pharynx, L4 animals were grown on NGM plates for 24 h at 20 °C before they were scored for germ-cell corpses. Only the posterior arms of intact gonads were scored. Blind tests were carried out in all germ-cell corpse quantification experiments.
Quantification of mitotic nuclei
L4 animals treated with 2.8 μM of purified tCPR-4 proteins in liquid culture for 48 h were transferred to NGM plates and allowed to recover for 1 h at 20 °C. They were then dissected to expose their gonads following the protocol described previously24. Dissected gonads were fixed and stained with DAPI34. The number of germ nuclei and the number of metaphase nuclei in the mitotic zone of each gonad were scored using a Zeiss Nomarski microscope with a DAPI filter24.
Quantification of the expression levels of cpr-4 through the GFP reporter
A single-copy insertion of the Pcpr-4::nls::gfp transgene32 was used to determine the expression levels of cpr-4 before and after irradiation. Middle stage L4 Pcpr-4::nls::gfp and cep-1(gk138); Pcpr-4::nls::gfp larvae were irradiated with 100 J m−2 UV and allowed to recover for 2 h at 20 °C before imaging. The GFP expression patterns of the animals were recorded by capturing images under Nomarski optics. The exposure times of all images were fixed at 100 ms. The intensity of GFP fluorescence in each animal was quantified using the Image J software (NIH). The expression levels of cpr-4 at different developmental stages (embryos, L1, L2, L3, L4 larvae, adults at 24 h and 48 h post L4) without irradiation were determined using the same method.
Embryonic lethality and larval arrest assays
For embryonic lethality assays caused by direct irradiation, after irradiated with 100 J m−2 UV or 500 Gy gamma ray, gravid adults were placed on NGM plates to lay eggs for 4 h at 25 °C and then removed from the plates. For embryonic lethality assays in liquid media, L4 larvae were cultured in conditioned medium or S-medium containing the purified proteins at 20 °C for 48 h, transferred to fresh NGM plates from the liquid media, and allowed to lay eggs for 4 h at 25 °C, before the adult animals were removed. For embryonic lethality assays in transgenic animals expressing CPR-4 in the pharynx, transgenic gravid adults at 24 h after L4 were placed on NGM plates to lay eggs for 4 h at 25 °C and then removed from the plates. In all cases, after 24 h at 25 °C on NGM plates, the number of eggs that did not hatch (scored as dead eggs) and the number of eggs that developed into larvae were scored and used to determine the rate of embryonic lethality.
For the larval arrest assays, gravid transgenic adults were placed on NGM plates, control RNAi plates, or cpr-4 RNAi plates to lay eggs for 4 h at 25 °C. The number of transgenic larvae that hatched out was scored under the fluorescence stereoscope before the plates were returned to the 20 °C incubator. After 3 days, the number of transgenic animals that did not enter the adult stages was scored and used to determine the rate of larval arrest.
Molecular biology
Full-length cpr-4 cDNA was amplified by PCR from a C. elegans cDNA library. The signal peptide of CPR-4 is predicted using the SignalP 3.0 Server35. To construct the pGEX4T-2-tCPR-4 plasmid, a cpr-4 cDNA fragment encoding residues 16–336 was PCR amplified from the full-length cpr-4 cDNA clone and subcloned into a modified pGEX4T-2 vector through its NdeI and XhoI sites, which has a PreScission Protease cleavage site LEVLFQGP inserted right after the glutathione S-transferase (GST) coding sequence. To make the pGEX4T-2-tCPR-4(C109A), pGEX4T-2-tCPR-4(H281A) and pGEX4T-2-tCPR-4(N301A) vectors, two-step PCR was used to generate the tCPR-4 cDNA fragment carrying the indicated mutation, which was subcloned into the same modified pGEX4T-2 vector through its NdeI and XhoI sites. To construct Pmyo-2::CPR-4::mCherry, Pmyo-2::tCPR-4::mCherry, Pmyo-2::CPR-4(C109A)::mCherry, Pmyo-2::CPR-4(H281A)::mCherry, and Pmyo-2::CPR-4(N301A)::mCherry expression vectors, the cDNA fragments encoding full-length CPR-4(C109A), CPR-4 (H281A) and CPR-4 (N301A) were first generated using a two-step PCR method. The DNA fragments encoding CPR-4::mCherry, tCPR-4::mCherry, CPR-4(C109A)::mCherry, CPR-4(H281A)::mCherry and CPR-4(N301A)::mCherry were similarly PCR amplified and subcloned into a modified pCFJ90 vector (Addgene) through its NheI sites.
To make the plasmid pCFJ151-Pcpr-4::cpr-4::flag for generating the single-copy integrated transgene, a cpr-4 genomic fragment (Pcpr-4::cpr-4::utr), containing 4,018 bp of the cpr-4 promoter sequence, 1,196 bp of the cpr-4 genomic coding sequence, and 2,267 bp of the cpr-4 3′ untranslated region (UTR), was excised from a fosmid WRM0619bH11 through digestion with PmlI and BssHII and then subcloned into a modified pCFJ151 plasmid through its BssHII site and a blunted AvrII site. This Pcpr-4::cpr-4::utr genomic fragment was then excised from the plasmid through AflII and NheI digestion and subcloned into a plasmid pSL1190 through its AflII and NheI sites. A Flag tag (DYKDDDDK) was inserted immediately after the cpr-4 coding region through the QuickChange method. The modified Pcpr-4::cpr-4::flag::utr genomic fragment was subcloned back to pCFJ151 through its AflII and NheI sites to obtain the plasmid pCFJ151-Pcpr-4::cpr-4::flag.
To construct the plasmid pSL1190-Pcpr-4::nls::gfp for single-copy insertion, a 4,114 bp fragment containing the cpr-4 promoter and the first 58 bp of the cpr-4 coding region, a 1,767 bp fragment containing the NLS::GFP coding sequence and the unc-54 3′ UTR, a 1,337 bp upstream homologous recombination fragment of the LGII Mos I site (ttTi5605) and a 1,418 bp downstream homologous recombination fragment of the LGII MosI site were ligated into the pSL1190 plasmid backbone through its PstI and BamHI sites using the Gibson ligation method.
To construct the plasmid for cpr-4 RNAi, full-length cpr-4 cDNA fragment was PCR amplified and subcloned into the pPD129.36 vector through its NheI and XhoI sites. All clones generated were confirmed by DNA sequencing.
Transgenic animals
Transgenic animals were generated using the standard protocol36. Pmyo-2::CPR-4::mCherry, Pmyo-2::tCPR-4::mCherry, Pmyo-2::CPR-4(C109A)::mCherry, Pmyo-2::CPR-4(H281A)::mCherry, or Pmyo-2::CPR-4(N301A)::mCherry was injected into ced-1(e1735); cpr-4(tm3718) animals at 20 ng μl−1 (for quantification of germ-cell corpses) or 2 ng μl−1 (for embryonic lethality and larval arrest assays) along with the pTG96 plasmid (at 20 ng μl−1) as a co-injection marker. The pTG96 plasmid contains a sur-5::gfp translational fusion that is expressed in many cells and in most developmental stages37. Single-copy insertion Pcpr-4::cpr-4::flag transgene and Pcpr-4::nls::gfp transgene were generated using a method described previously32.
Immunoblotting detection of secreted CPR-4::Flag
Conditioned medium derived from irradiated N2, Pcpr-4::cpr-4::flag, Pcpr-4::cpr-4::flag; cpr-4(tm3718), cep-1(gk138); Pcpr-4::cpr-4::flag, or Pcpr-4::cpr-4::flag; daf-2(e1370); cpr-4(tm3718) animals was concentrated using a 10-kDa molecular mass cut-off centrifugal filter column (1 μg μl−1 final protein concentration). Concentrated conditioned media were resolved by 12% SDS–PAGE and transferred to a PVDF membrane. Secreted CPR-4::Flag was detected using a monoclonal antibody to the Flag tag (Sigma, F3165, 1:2,000 dilution) and a goat-anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP, Bio-Rad, 1705047, 1:5,000 dilution).
CPR-4::Flag depletion
UV-CM or UV-ctrl derived from Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals were incubated with 20 μl bed volume anti-Flag M2 affinity gel (Sigma, A2220) overnight at 4 °C on a rotary shaker. The anti-Flag beads were spun down by centrifugation at 10,000 r.p.m. for 2 min and the supernatant was collected and used as anti-Flag-depleted conditioned medium.
Protein expression and purification
tCPR-4 or mutant tCPR-4 proteins (C109A, H281A or N301A) were expressed in the E. coli strain BL21(DE3) with a N-terminal GST tag and a C-terminal His6-tag. The soluble fraction of bacteria was purified using a glutathione sepharose column (GE Healthcare, 17-0756-01) and cleaved by the PreScission Protease at room temperature for 2 h to remove the GST tag. The proteins were then affinity purified using a Ni2+ Sepharose column (GE Healthcare, 17-5268-01) and eluted from the column with 250 mM imidazole. Purified proteins were concentrated using 5 kDa molecular mass cut-off centrifugal filter units to approximately 200 ng μl−1 final concentration and dialysed twice using a dialysis buffer containing 25 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1mM DTT and 10% (v/v) glycerol at 4 °C for 4–6 h with magnetic stirring. Insoluble aggregates after dialysis were removed by high-speed centrifugation. The proteins were then diluted to 100 ng μl−1 final concentration with the dialysis buffer and stored at −80 °C in aliquots. The concentrations of purified proteins were determined by anti-His6 immunoblotting, using tCPR-4–His6 with a known concentration as a normalizing control.
Mass spectroscopy analysis
The protein bands of interests excised from the silver-stained gels were destained by 1% potassium ferricyanide and 1.6% sodium thiosulfate, subjected to reduction and alkylation by 10 mM DTT and 55 mM iodoacetamide in 25 mM NH4HCO3, and then in-gel digested with trypsin (20 μg ml−1 in 25 mM NH4HCO3) at 37 °C for 16 h. The reaction products were analysed with liquid chromatography tandem mass spectrometry (LC–MS/MS) using a linear ion trap mass spectrometer (LTQ-Orbitrap, Thermo Fisher). Samples were loaded across a trap column (Zorbax 300SB-C18, 0.3 × 5 mm, Agilent Technologies) and peptides were separated on an analytical column (capillary RP18 column, Synergy hydro-RP, 2.5 μm, 0.075 × 100 mm, packed in house) with a gradient of 2–95% HPLC buffer (99.9% acetonitrile containing 0.1% formic acid) in 75 min. For the MS analysis, we used a data-dependent procedure that alternated between one MS scan and six MS/MS scans for the six most abundant precursor ions. The resulting spectra were used in searches of the sprot_20140416 database (selected for C. elegans, 3,466 entries) assuming the digestion enzyme trypsin. The MASCOT search engine (http://www.matrixscience.com; v.2.2.2 Matrix Science) was used, allowing two missing cleavage sites with charge states from 2+ to 3+. The parent ion mass tolerance was set to 10 p.p.m. and the fragment ion mass tolerance was set to 0.5 Da for both fix modification (carbamidomethylation of cysteine) and variable modifications (acetylation at protein N-terminal, oxidation of methionine, and Gln change to pyro-Glu). The DAT files produced by Mascot Daemon were subjected to search using Scaffold 3 search engine (v.3.06.01; http://www.proteomesoftware.com). Protein identification is accepted if protein probability is >95%, containing at least two peptides with peptide prophet algorithm probability >95%.
Measurement of protease activity in vitro
The CPR-4 enzymatic assays were performed following the method described previously with some modifications38. The cathepsin B-specific fluorogenic substrate, Z-Arg-Arg-7-amido-4-methylcoumarin hydrochloride (z-Arg-Arg-AMC; Peptanova, 88937-61-5), was dissolved in 2× reaction buffer, containing 25 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10% (v/v) glycerol, 0.8 mM sodium acetate (pH6.0), and 8 mM EDTA. For the assays, 10 μl of proteins (100 ng μl−1) or 10 μl of conditioned medium (100 ng μl−1) were incubated with 10 μL of 20 μM z-Arg-Arg-AMC at 25 °C for 10 min before measuring the luminescence. Enzymatic activities were determined as the mean velocities at 25 °C in a dual luminescence fluorometer EnVision (Perkin-Elmer) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm, and expressed as relative intensity in RFUs. rhCTSB (Sino Biological Inc., 10483-H08H-10) was dissolved in a buffer recommended by the manufacturer (25 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10% (v/v) glycerol, 5 mM DTT, and 0.1% Triton-X). The buffer control unique to the CPR-4 proteins or the rhCTSB protein, or the sham-irradiated conditioned medium, was also measured using the same procedures. S-medium was used in each experiment as the background control.
qRT–PCR analysis of the cpr-4 transcriptional levels
N2 and cep-1(gk138) L4 larvae were transferred to fresh NGM plates and cultured at 20 °C for 24 h. Two hours after they were subjected to 100 J m−2 UV or 500 Gy gamma ray irradiation or sham-irradiation, they were lysed for total RNA extraction using the RNAiso kit (TaKaRa, 9108). Isolated total RNAs were used as templates in reverse transcription using the ImProm-II Reverse Transcription System (Promega, A3800) to obtain the first-strand cDNA according to manufacturer’s instructions.
Quantitative PCR analysis was carried out using a Bio-Rad CFX96 Touch real-time PCR detection system using the iTaq SYBR Green Supermix with ROX (Bio-Rad, 1725151). Each PCR reaction contained 12.5 μl of the Bio-Rad supermix solution, 50 nM of forward and reverse primers, and 5 μl cDNA (150 ng μl−1) in a final volume of 25 μl. Amplifications were performed in real-time PCR tubes (Bio-Rad, TLS0851) placed in the 96-well of the real-time PCR detection system. The cycling conditions were as follows: 95 °C for 3 min for denaturation, followed by 50 cycles of 20 s at 95 °C, 30 s at 60 °C, and 20 s at 72 °C. Melting curve analysis was performed after the final cycle to examine the specificity of primers in each reaction. PCR reactions were run in triplicate and three independent experiments were performed. The transcription of pmp-3 was used as the internal reference due to its unusually stable expression levels in adults39. The data were analysed by the Livak method. The primers to detect cpr-4 are 5′-TCGGAAAGAAGGTCTCCCAGAT-3′ (forward) and 5′-GGTAGAAGTCCTCGTAGACAGTGAAT-3′ (reverse). The primers to detect pmp-3 are 5′-GTTCCCGTGTTCATCACTCAT-3′ (forward) and 5′-ACACCGTCGAGAAGCTGTAGA-3′ (reverse).
Data availability
The uncropped versions of the blots are provided in Supplementary Fig. 1. Most of the raw data for the figures and tables presented in this paper are also provided in the Source Data. The other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mothersill, C. & Seymour, C. Radiation-induced bystander effects: past history and future directions. Radiat. Res. 155, 759–767 (2001)
Prise, K. M. & O’Sullivan, J. M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 9, 351–360 (2009)
Rzeszowska-Wolny, J., Przybyszewski, W. M. & Widel, M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur. J. Pharmacol. 625, 156–164 (2009)
Stergiou, L., Doukoumetzidis, K., Sendoel, A. & Hengartner, M. O. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 14, 1129–1138 (2007)
Derry, W. B., Putzke, A. P. & Rothman, J. H. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294, 591–595 (2001)
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001)
Klokov, D. et al. Low dose IR-induced IGF-1-sCLU expression: a p53-repressed expression cascade that interferes with TGFβ1 signaling to confer a pro-survival bystander effect. Oncogene 32, 479–490 (2013)
Koturbash, I. et al. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int. J. Radiat. Oncol. Biol. Phys. 70, 554–562 (2008)
Sun, Y. et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 18, 1359–1368 (2012)
Buck, M. R., Karustis, D. G., Day, N. A., Honn, K. V. & Sloane, B. F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 282, 273–278 (1992)
Poole, A. R., Tiltman, K. J., Recklies, A. D. & Stoker, T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature 273, 545–547 (1978)
Larminie, C. G. & Johnstone, I. L. Isolation and characterization of four developmentally regulated cathepsin B-like cysteine protease genes from the nematode Caenorhabditis elegans. DNA Cell Biol. 15, 75–82 (1996)
Lorimore, S. A., Rastogi, S., Mukherjee, D., Coates, P. J. & Wright, E. G. The influence of p53 functions on radiation-induced inflammatory bystander-type signaling in murine bone marrow. Radiat. Res. 179, 406–415 (2013)
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002)
Bertucci, A., Pocock, R. D., Randers-Pehrson, G. & Brenner, D. J. Microbeam irradiation of the C. elegans nematode. J Radiat. Res. 50, A49–A54 (2009)
Mort, J. S. & Buttle, D. J. Cathepsin B. Int. J. Biochem. Cell Biol. 29, 715–720 (1997)
Shore, D. E. & Ruvkun, G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 23, 409–420 (2013)
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010)
Perrin, A. J. et al. Noncanonical control of C. elegans germline apoptosis by the insulin/IGF-1 and Ras/MAPK signaling pathways. Cell Death Differ. 20, 97–107 (2013)
Scott, B. A., Avidan, M. S. & Crowder, C. M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296, 2388–2391 (2002)
Paradis, S., Ailion, M., Toker, A., Thomas, J. H. & Ruvkun, G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13, 1438–1452 (1999)
Lin, K., Dorman, J. B., Rodan, A. & Kenyon, C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278, 1319–1322 (1997)
Ogg, S. et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997)
Michaelson, D., Korta, D. Z., Capua, Y. & Hubbard, E. J. Insulin signaling promotes germline proliferation in C. elegans. Development 137, 671–680 (2010)
Pinkston, J. M., Garigan, D., Hansen, M. & Kenyon, C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313, 971–975 (2006)
Nikjoo, H. & Khvostunov, I. K. A theoretical approach to the role and critical issues associated with bystander effect in risk estimation. Hum. Exp. Toxicol. 23, 81–86 (2004)
Mothersill, C. & Seymour, C. Radiation-induced bystander and other non-targeted effects: novel intervention points in cancer therapy? Curr. Cancer Drug Targets 6, 447–454 (2006)
Recklies, A. D., Tiltman, K. J., Stoker, T. A. & Poole, A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 40, 550–556 (1980)
Barrett, A. J. & Kirschke, H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 80, 535–561 (1981)
Shree, T. et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 (2011)
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974)
Frøkjær-Jensen, C. et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40, 1375–1383 (2008)
Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001)
Pepper, A. S. R., Killian, D. J. & Hubbard, E. J. A. Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163, 115–132 (2003)
Bendtsen, J. D., Nielsen, H., von Heijne, G. & Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795 (2004)
Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991)
Gu, T., Orita, S. & Han, M. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 18, 4556–4564 (1998)
Paquet, C., Sané, A. T., Beauchemin, M. & Bertrand, R. Caspase- and mitochondrial dysfunction-dependent mechanisms of lysosomal leakage and cathepsin B activation in DNA damage-induced apoptosis. Leukemia 19, 784–791 (2005)
Hoogewijs, D., Houthoofd, K., Matthijssens, F., Vandesompele, J. & Vanfleteren, J. R. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol. Biol. 9, 9 (2008)
Acknowledgements
We thank J. Tyler for help with localized irradiation experiments and T. Su for discussion. This work was supported by National Basic Research Program of China (2013CB945602), National Scientific and Technological Major Project of China (2013ZX10002-002), fellowships from China Scholarship Council and Fujian Agriculture and Forestry University (L.Z.) and Tsinghua University-Peking University Center for Life Sciences (Q.L. and X.Z.), and NIH grant R35 GM118188 (D.X.).
Author information
Authors and Affiliations
Contributions
Y.P. set up conditioned medium RIBE assays and performed fractionation, RNAi screen, protein purification, and protease assays. M.Z. and Q.L. generated transgenic animals and conducted experiments with Y.P., aided by H.G. and X.Z. H.L. and L.Z. set up intra-animal RIBE assays and collected related data, aided by Y.-Z.C., X.W. and B.L. J.-T.C. and J.-S.Y. performed mass spectrometry analysis. S.Y. and S.M. generated tm3718. D.X. conceived and supervised the project, analysed data, and wrote the manuscript together with Y.P., M.Z., L.Z., Q.L. and H.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Dillin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Conditioned medium generated from UV or IR and purified tCPR-4 proteins cause embryonic lethality.
a, The embryonic lethality rate of wild-type (N2) or cep-1(gk138) animals after 100 J m−2 UV irradiation or 500 Gy IR compared with sham-irradiation controls. b, N2 animals were used to generate UV-CM, UV-ctrl, IR-CM and IR-ctrl, which were used to treat unexposed N2 animals in the embryonic lethality assays (Methods). c, Recombinant tCPR-4 (wild type or mutant; 2.8 μM), recombinant human cathepsin B (rhCTSB; 0.27 μM) or the buffer control were used to treat N2 animals in the embryonic lethality assays. Total numbers of embryos scored: 1,781, 805, 1,249, 2,645, 596 and 1,862 embryos, from the left to the right in a; 2,721, 2,484, 880 and 743, from left to right in b; and 979, 875, 929, 939, 907 and 777, from left to right in c. Six independent assays (a, UV-ctrl and UV-CM in b) and three independent assays (IR-ctrl and IR-CM in b, c) were performed for each condition. Data are mean ± s.e.m. NS, not significant; **P < 0.01, ***P < 0.001, two-sided, unpaired t-test.
Extended Data Figure 2 Characterization of the nature and the source of the RIBE factors.
a, b, Treatment of UV-CM and UV-ctrl collected from N2 animals irradiated at 100 J m−2 with RNase (1 μg μl−1) or DNase (0.01 U μl−1) did not alter the apoptosis-inhibitory effect on ced-1(e1735) animals (Methods). Germ-cell corpses were scored after 48-h treatment of ced-1(e1735) L4 larvae. c, e–g, ced-1(e1735) L4 larvae were treated with UV-CM and UV-ctrl (0.1 μg μl−1) prepared from ced-3(n2433) animals (c), glp-1(e2141ts) animals grown at 25 °C (e), N2 animals fed with formaldehyde-treated HB101 bacteria (f), and Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals with or without anti-Flag depletion (g), respectively. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars (a–c, e–g). **P < 0.01, ***P < 0.001, two-sided, unpaired t-test. d, Representative differential interference contrast (DIC) images (at least 10) of N2 and glp-1(e2141) adult animals grown at 25 °C. The gonads of the N2 animal with multiple oocytes and fertilized eggs are outlined with dash lines. glp-1(e2141) animal had no visible germ line. Scale bars, 100 μm. h, Immunoblotting analysis of secreted CPR-4::Flag in UV-CM and UV-ctrl prepared from Pcpr-4::cpr-4::flag; cpr-4(tm3718) animals with or without anti-Flag depletion treatment. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 3 Identification of CPR-4 as the RIBE factor.
a, Full medium, the >10 kDa fraction, and the <10 kDa fraction of UV-CM and UV-ctrl derived from N2 animals were used to treat ced-1(e1735) animals in germ-cell corpse assays as in Fig. 1b. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. b, Identification of CPR-4 as the RIBE factor through the RNAi screen. UV-ctrl and UV-CM prepared from RNAi-treated animals were used to treat ced-1(e1735) animals. The number of germ-cell corpse decrease (y-axis) was calculated by subtracting the number of average germ-cell corpses under UV-ctrl treatment from that under UV-CM treatment. Among the candidate genes, RNAi of eft-3, ubq-2 and act-1 caused strong embryonic lethality and we were unable to obtain their UV-CM. RNAi of his-1, his-4 and his-71 caused partial embryonic lethality. 20 gonad arms were scored in each RNAi experiment. c, Secretion of CPR-4::Flag into UV-CM was greatly reduced in irradiated cep-1(gk138) animals carrying a single-copy integration of Pcpr-4::cpr-4::flag compared with that from irradiated N2 animals carrying the same Pcpr-4::cpr-4::flag transgene. Concentrated UV-CM or UV-ctrl (1 μg μl−1) from the indicated strains was subjected to the immunoblotting analysis using an antibody to the Flag epitope. d, The protease activity of 0.27 μM rhCTSB or 2.8 μM recombinant tCPR-4 protein was measured as in Fig. 2b. Data are mean ± s.e.m. (n = 6 in each assay). e, rhCTSB (0.27 μM) or the buffer control was used to treat ced-1(e1735) animals. Animals cultured in the rhCTSB buffer grew slower than in the tCPR-4 buffer and had less germ-cell corpses. Data are mean ± s.e.m. (n = 21 in each assay). Germ-cell corpses were scored after 48 h treatment (a, b, e). ***P < 0.001, two-sided, unpaired t-test. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 4 Representative MS/MS spectra from LTQ-Orbitrap used to confirm the identity of CPR-4 in UV-CM.
a, Tryptic peptides of protein band 6 in the SDS–PAGE gel (Fig. 1d) were analysed by LC–MS/MS using LTQ-Orbitrap. The amino acid sequences of peptides identified by MS/MS analysis and matched to the amino acid sequences of CPR-4 are underlined and in red. b, The MS/MS spectra of the two peptides identified in a are shown. The assignments of the fragmented ions observed to specific amino acid residues were performed using the Scaffold 3 search engine, and the search results are shown below the MS/MS spectra. The lower-case ‘c’ indicates the carbamidomethyl-modified cysteine residue in the tryptic peptide.
Extended Data Figure 5 The cpr-4 deletion mutation and sequence alignment of human and mouse cathepsin B and CPR-4.
a, A schematic representation of the cpr-4 gene structure and the tm3718 deletion. Exons are depicted as blue boxes and introns and the untranslated region as lines. The red box indicates the region of cpr-4 removed by the 406-bp tm3718 deletion. The green box indicates a 12-bp insertion. b, Sequence alignment of human cathepsin B, mouse cathepsin B and CPR-4. Residues that are identical in all three proteins are shaded in pink. The two catalytic residues are shaded in green, which are the active-site cysteine residue that serves as a nucleophile and the histidine residue that acts as a general base to facilitate hydrolysis of the peptide bonds of the substrates16, respectively.
Extended Data Figure 6 Analysis of the roles of additional genes in mediating RIBEs.
a, LUI assays. Animals of the indicated genotype were analysed for the bystander Phsp-4::gfp response 24 h after localized irradiation at the head region as described in Fig. 3. Data are mean ± s.e.m. The numbers of animals scored are indicated inside the bars. b, Germ-cell corpse assays after tCPR-4 treatment. 2.8 μM recombinant tCPR-4 protein or buffer control was used to treat L4 larvae of the indicated genotype as described in Fig. 4a. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. c, Immunoblotting analysis of secreted CPR-4::Flag in UV-CM and UV-ctrl from Pcpr-4::cpr-4::flag; daf-2(e1370); cpr-4(tm3718) animals was done as in Fig. 1f. d, Germ-cell corpse assays. ced-1(e1735) L4 larvae were treated with UV-CM and UV-ctrl (0.1 μg μl−1) prepared from c. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. e, Germ-cell proliferation assays. N2 and cep-1(gk138) L4 larvae were treated in S-medium containing 2.8 μM recombinant tCPR-4 or buffer control for 48 h. The numbers of nuclei and metaphase nuclei in the mitotic zone of the germ line were scored (Methods). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, two-sided, unpaired t-test. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 7 The expression patterns of cpr-4 in C. elegans.
a–g, i, Representative GFP and DIC images (at least 15 each) of N2 animals (a–g) or cep-1(gk138) animals (i) carrying a single-copy integration of Pcpr-4::nls::gfp at the indicated developmental stages. Arrows point to the embryo and the L1 larva that showed no or very dim GFP (a, b). Scale bar, 100 μm. h, Representative DIC, GFP and DIC and GFP merged images (at least 15) of a L4 larva carrying the same Pcpr-4::nls::gfp transgene (left), and corresponding tenfold magnified images showing GFP expression in intestinal cells (right). GFP was seen mostly in the nuclei (arrows). Scale bars, 100 μm (left) and 10 μm (right). j, The intensity of GFP fluorescence in Pcpr-4::nls::gfp and cep-1(gk138); Pcpr-4::nls::gfp animals at different developmental stages was quantified using the Image J software. Data are mean ± s.e.m. n = 28, 28, 24, 31, 30, 33, 52, 52, 19, 28, 52, 52, 24 and 25 animals, scored from left to right, respectively. **P < 0.01, ***P < 0.001, two-sided, unpaired t-test. k, Quantification of GFP intensity in N2 and cep-1(gk138) animals carrying the same single-copy Pcpr-4::nls::gfp transgene irradiated by UV or sham-irradiated, using Image J. Data are mean ± s.e.m. n = 38, 37, 32 and 30 animals, scored from left to right, respectively. ***P < 0.001, two-sided, unpaired t-test.
Extended Data Figure 8 Pharyngeal expression of CPR-4 results in embryonic lethality, larval arrest and reduced germ-cell death.
a, b, Representative DIC and mCherry images (at least 10) of adult animals with pharyngeal expression of CPR-4::mCherry (a) and tCPR-4::mCherry (b). White dashed lines highlight the edge of the pharynx. Arrowheads indicate cells, including coelomocytes, that had taken up CPR-4::mCherry (a), which was made in and secreted from the pharynx and transported to other parts of the animal, probably through the pseudocoelom, a fluid-filled body cavity. The enlarged images of two pairs of posterior cells with weak fluorescence (indicated by colour arrowheads) are shown in dashed boxes with corresponding colours. Scale bars, 100 μm. c, The percentages of embryonic lethality and larval arrest were scored in embryos or larvae carrying Pmyo-2::CPR-4::mCherry (wild-type or mutant) or Pmyo-2::tCPR-4::mCherry transgenes. Three independent transgenic lines were scored for each construct. The number of newly hatched transgenic L1 larvae scored and the number of transgenic embryos scored are indicated in parentheses. The increased larval arrest seen in Pmyo-2::CPR-4::mCherry transgenic animals was blocked when transgenic animals were treated with cpr-4 RNAi (Extended Data Table 2), indicating that reducing cpr-4 expression prevents larval arrest. All animals carry the ced-1(e1735) and cpr-4(tm3718) mutations (a–c). d, Quantification of germ-cell corpses in transgenic animals. L4 ced-1(e1735); cpr-4(tm3718) animals carrying the indicated transgenes were grown on regular NGM plates for 24 h before examination. Data are mean ± s.e.m. The numbers of gonad arms scored are indicated inside the bars. ***P < 0.001, one-way analysis of variance (ANOVA).
Supplementary information
Supplementary Figure
This file contains full scans of the gel images shown in the main and Extended Data Figures.
Source data
Rights and permissions
About this article
Cite this article
Peng, Y., Zhang, M., Zheng, L. et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature 547, 458–462 (2017). https://doi.org/10.1038/nature23284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23284
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.