Abstract
Introduction
Patients with severe neurogenic bowel dysfunction (NBD) may undergo the Malone antegrade continence enema (MACE) surgery to perform antegrade bowel irrigation (ABI). The standard approach may be prevented by a previous appendectomy or complicated by appendicular stenoses and/or stomal leakages. We present the experience by our tertiary referral center for NBD, adopting a modified surgical technique, based on a neoappendix with the terminal ileum to preserve the natural anti-reflux mechanism of the ileocecal valve and avoid stool leakage, and a largely available transanal irrigation (TAI) system to catheterize the neoappendix and perform ABI.
Case presentation
Three individuals with NBD successfully underwent our modified MACE program. Case 1 had cauda equina syndrome. He underwent surgery at 40. Case 2 was a man who suffered from spinal cord dysfunction due to acute disseminated encephalomyelitis, functionally T12 AIS B, at 57. Case 3 was a man with traumatic L1 AIS B paraplegia. At 60 he underwent surgery after 29 years since the injury. He needed a surgical revision due to a postoperative subcutaneous infection. After 121, 84 and 14 months from surgery, the three individuals performed ABI every 2 days, presented functional stomas, had no fecal incontinence, and reported an NBD score of 6, compared to 40, 33 and 35 pre-operatively.
Discussion
To our knowledge, this is the first report of MACE combining a tapered terminal ileum conduit and an adapted TAI system. Our approach proved to be a safe and effective strategy for severe NBD avoiding a colostomy.
Similar content being viewed by others
Introduction
Neurogenic bowel dysfunction (NBD) is a condition encompassing chronic constipation and fecal incontinence (FI) that occurs after spinal cord injury (SCI) [1]. NBD represents a significative impairment with a negative impact on the quality of life (QoL) [2].
NBD may be successfully treated with conservative approaches (i.e., appropriate diet, oral laxatives, suppositories). However, in case of failure a transanal irrigation (TAI) program may help achieve a suitable bowel management program in most cases [3]. When NBD is unresponsive to the previous approaches, a colostomy may be considered. This surgery may be avoided by performing a Malone antegrade continence enema (MACE), which is a surgical procedure combining the principles of antegrade colonic washout and the Mitrofanoff non-refluxing catheterisable channel [4]. The result is a continent catheterisable stoma to perform antegrade bowel irrigation (ABI), achieving complete colonic emptying and preventing fecal soiling.
The MACE involves the creation of a neostoma with the appendix anastomosed to the abdominal wall creating a valve mechanism that enables appendix catheterization and avoids stool leakage from the stoma. When the appendix is unusable and/or absent (i.e., due to surgery), a neoappendix may be created with a tubularized cecal flap and/or a tapered terminal ileum conduit [5, 6]. Still, the morbidity with these procedures may be significant, including fecal leakage and/or (neo)appendicular stenosis with subsequent difficulties with the catheter introduction [7].
To overcome these problems, we used a novel MACE surgical approach, derived from the terminal ileum conduit technique originally described by Christensen et al. (2001) [8]. Subsequently, we recommended patients perform anterograde bowel irrigation (ABI) using a modified TAI system.
The aim of our study was to report the initial experience with our MACE approach in NBD by a tertiary referral center.
Methods
In March 2020, we retrospectively collected data of our patients affected by NBD in SCI, who underwent the MACE surgical procedure from January 2010 to December 2019.
We included the following pre-operative data (Appendix 1): sex, etiology of NBD, age at surgery, time lapse from NBD diagnosis to surgery. We used the NBD score (range: 0–47) to examine QoL related to bowel symptoms and sensitivity to treatment change [9]. We also used the American Society of Anesthesiologists (ASA) score and the Charlson comorbidity index (CCI) to estimate the anesthesiologic risk (range: I–VI) and the comorbidity burden (range: 0–37), respectively [10, 11].
We screened for the following surgical-related data (Appendix 2): concomitant surgical procedures, operative time (OT), estimated blood loss (EBL), intra-operative complications, hospital stay, early postoperative complications estimated within 1 month from surgery. We classified surgical-related complications following the Clavien–Dindo system (grades: I–V) [12]. Since some surgeries were accompanied by other procedures (i.e., cholecystectomy), we isolated the effective OT to perform the MACE procedure.
We assessed the patient’s status at the last follow-up visit (Appendix 3) focusing on the stoma trophism, coprostasis signs (i.e., impaction of feces in the gastrointestinal tract) under abdominal x-ray and the bowel emptying through detailed history and NBD score.
Data were stored anonymously using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The reduced population sample did not enable any descriptive and inferential statistics.
We conducted this study in accordance with the Declaration of Helsinki.
Surgical procedure
An expert general surgeon carried out all the procedures under general anesthesia. We performed a right pararectal incision of about 6 cm. We mobilized the coecum. Paying attention to the related independent vascularization, we isolated the last part of the terminal ileum about 15 cm before the ileocecal valve to preserve continence (Fig. 1). We divided the ileum with a 75 mm linear stapler. We inserted a 14 Fr Nelaton catheter and tapered the caliber of the distal ileum along the antimesenteric border by mechanical sutures. We configured manually a novel side-to-side ileo-colonic anastomosis at about 20 cm from the coecum using a double-layer technique. We solidarized the defunctionalized bowel loop to the back of the abdominal wall and created a stoma in the right iliac fossa using absorbable sutures. After accurate hemostasis, we performed the layered closure and intradermal suture. At the end of the procedure, we left a 14 Fr Foley catheter in situ for 2 weeks with the balloon inflated after the ileocecal valve.
After a right pararectal incision, we isolated the last part of the distal terminal ileum and divided the ileum with a 75 mm linear stapler about 15 cm before the ileocecal valve. We inserted a 14 Fr Nelaton catheter and reduced the calibre of the distal ileum along the antimesenteric border by a linear stapler. We configured a novel side-to-side ileo-colonic anastomosis using a double-layer technique and created a skin stoma in the right iliac fossa using absorbable sutures. We performed a layered closure and left a 14 Fr Foley catheter in situ for 2 weeks with the balloon cuffed after the ileocecal valve.
Post-operative management and follow-up
In the early postoperative phase, all patients took oral laxatives and suppositories. After 2 weeks, we addressed all cases to our ABI program, which was similar to the well-established bowel program for TAI [13].
To start with, we modified the largely available and well-acknowledged TAI system, called Peristeen™ (Coloplast™, Humlebæk, Denmark). We adapted the connector to a 14 Fr Nelaton catheter to perform pressure-controlled ABI (Fig. 2).
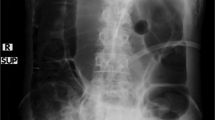
We adapted a largely available transanal irrigation system to perform ABI. The white liquid in the bag consisted of barium sulfate diluted in saline (temperature = 35–37 °C). We adopted the system connector to a 14 Fr Nelaton catheter, inserted through the neoappendix. We pumped the solution progressively and performed pressure-controlled ABI. Since the catheter was not blocked with a balloon, the patient should keep manually the catheter in situ. We monitored the fecal bulk with the contrast medium under fluoroscopic guidance. We noticed the beginning of bowel evacuation with a liquid volume equal to 600 ml. After evacuation, we checked for the complete bowel emptying radiographically.
During the training, we filled the system bag with 150–200 g of barium sulfate diluted to 2 L of saline to visualize and control the bowel emptying under fluoroscopic guidance. We progressively inserted the liquid (temperature = 35–37 °C) into the bowel up to the patient’s evacuation. We performed a fluoroscopic control every 100 mL of solution. During these tests the patients were supine; however, they performed ABI on the toilet at home.
We invited all patients to take oral laxatives, like polyethylene glycol (PEG), to soften feces with water irrigation.
Based on our TAI program, we performed the first ABI under our supervision. Then, we invited patients to perform it independently for 15 days. During the second visit we controlled the ABI execution, assessed the evacuation fluoroscopically and corrected any mistakes during the procedure to ensure a complete and safe bowel emptying. If the patients learned to perform ABI successfully and safely, the following follow-up visits were planned 3, 6, 12 months after surgery and, later, annually.
During each follow-up visit, we performed a physical examination paying attention to the stoma trophism. We assessed bowel management, asking for the ABI timing, success of ABI to induce bowel evacuation, spontaneous bowel evacuations, episodes of stool/mucus/blood leakage, any difficulties with catheter insertion. We screened for the chronic use of oral laxatives and/or suppositories. Moreover, we administered the NBD questionnaire.
Considering the strict relationships among all sacral functions (i.e., bowel, bladder and genitosexual ones), we investigated also sexual life and bladder management, focusing on urinary incontinence (UI) and symptomatic urinary tract infections (UTIs).
During the last follow-up visit, we estimated the spinal cord independence measure (SCIM) for each individual (range: 0–100) [14].
Case 1
In our first case, a 40-year-old male had a 1-year history of cauda equina syndrome, S1 ASIA impairment scale grade (AIS) C. He entered our hospital because of Fournier’s gangrene, requiring surgical debridement of the perianal, scrotal, left inguinal, iliac and lumbar regions. Later, he suffered from severe NBD (NBD score = 40). We could not create a standard colostomy due to the involvement of the left iliac quadrant. Therefore, we opted to perform MACE surgery. The ASA score was IV due to Fournier’s gangrene during the previous month. Neither intra- nor postoperative complications occurred. However, he required a long hospitalization (41 days) for the concomitant treatment of Fournier’s gangrene with antibiotics and hyperbaric oxygen therapy.
Case 2
He was a man who developed NBD at the age of 57 because of a spinal cord dysfunction, functionally T12 AIS B, due to acute disseminated encephalomyelitis (ADEM). After trying pharmacological therapy and TAI without success for a year, we opted for MACE surgery, but he did not have an appendix for the standard procedure, as it was surgically removed in the previous years. The procedure lasted longer than other cases (275 min) because of concomitant surgeries (colostomy reversal and abdominoplasty with laparocele correction). It was uneventful, and the individual had a short hospital stay (8 days). He was the only case to start the ABI program as an out-patient.
Case 3
This individual experienced a traumatic SCI, L1 AIS B, when he was 31 years old. He became unresponsive to medicines and TAI 29 years post injury. His relatively young age and few comorbidities (CCI: 3) made us consider the Malone procedure rather than a colostomy. The OT was the shortest: 160 min. The EBL was in line with others (about 350 ml). However, on the 8th postoperative day, he needed a surgical revision due to a subcutaneous infection resolved with drainage and antibiotics. We performed this procedure under general anesthesia (complication classified as Grade IIIb). He demanded a long-course antibiotic treatment requiring a long hospital stay (22 days).
Follow-up visits
Our patients attended their last follow-up visit 14 (Case #1), 84 (Case #2) and 121 (Case #3) months after surgery. Since surgery all cases had been performing ABI on the toilet every 2 days (success rate: 100%). The patients used the same amount of saline (600 ml) estimated during the training phase. They self-catheterized and performed enemas alone. All patients took PEG daily and suppositories on demand. Time spent on each evacuation was <30 min. No patient reported FI, bowel bleeding episodes, uncontrollable flatus, difficulties with the catheter insertion. They did not register uneasiness, sweating and/or headaches during or after evacuation. The last NBD score was 6 in 3/3 cases. During the last physical examination, all stomas were continent, functional and without any signs of stenosis and/or necrosis of the flap. During each follow-up visit, we performed plain abdominal radiographs: they documented minimal meteorism (i.e., accumulation of gas in the gastrointestinal tract) without significant coprostasis, proving a successful bowel emptying.
As for bladder management, all patients performed clean intermittent catheterizations, while Cases 2 and 3 took also antimuscarinic therapy (AMT). We registered neither UI nor symptomatic UTIs. All cases suffered from erectile dysfunction (ED), but they achieved regular sexual intercourse with phosphodiesterase type 5 inhibitors on demand.
At the last follow-up visit, the SCIM was 93 in Case #1 (self-care = 20; respiration and sphincter management = 35), 63 in Case #2 (self-care = 20; respiration and sphincter management = 30), and 72 in Case #3 (self-care = 20; respiration and sphincter management = 35).
Discussion
Bowel dysfunction may manifest as chronic constipation and/or inability to control stool elimination: according to the adopted definition, it may affect up to a quarter of the general adult population [15]. FI affects old people the most, representing one of the pressing challenges for the national healthcare systems in terms of costs (i.e., need for pads) and morbidities (i.e., surgery, risk of falls) [16, 17]. Similarly, young people with neurological deficits may suffer from NBD and they usually report NBD as more problematic than any other impairments [18,19,20].
Our NBD treatment is based on a stepwise approach to ensure toileting in efficient time, to avoid FI and to minimize the QoL impairment secondary to the bowel management plan [21]. Conservative management is based upon a diet rich in both fiber and water and a well-defined bowel routine. We advise all our patients to take oral laxatives (PEG) once per day or every 2 days. If necessary, we suggest suppositories of glycerin (lubricant) or bisacodyl (stimulant). Healthcare professionals should assure concomitant medications (e.g., AMT) do not worsen NBD [22].
According to the patient’s hand function and toileting independence, many adjuvant techniques may assist bowel evacuation: abdominal massage, Valsalva maneuver, digital rectal stimulation and/or evacuation. We discourage digital rectal evacuation and support the use of bowel micro-enemas to start defecation and avoid anorectal lesions.
Severe NBD may require TAI from the very beginning to limit several complications, like FI episodes, need for stoma, UI, and UTIs [23]. The last choice for people experiencing unresponsive NBD is represented by colostomies with high successful rates. However, different-graded complications may occur: leakage of mucus and occasionally blood/pus per rectum, parastomal hernias and bowel obstruction. In addition, the colostomy may be associated with poor bowel emptying in patients with reduced colonic motility. Right-sided colostomy may solve this limit, but it is associated with more liquid stools, increased stoma care requirements, and greater risk of leaks.
In 1990, Malone et al. reported an innovative technique to overcome these problems [24]. The largest published series included 300 pediatric patients undergoing MACE: the overall success rate was 79% after a mean follow-up of 2.4 years [25]. The main complications were stomal stenoses (30%) and stomal leakages (7%). Conversely, poor results proved to be associated with adults [26]. After a mean follow-up of 6.6 years, only 8/16 patients used the MACE successfully without experiencing FI and/or constipation, due to a specific subgroup (chronically constipated patients without fecal soiling), technical problems and difficulties with patient compliance.
To overcome all the above-mentioned issues, we developed our MACE program, encompassing a tapered terminal ileum conduit, a largely available TAI system and a well-defined follow-up strategy. We identified several strengths of our workflow. Our surgical approach was based on the preservation of the ileocecal valve to avoid fecal leakage. The bowel integrity was largely preserved without impairing an optimal fecal formation. The manual double-layer side-to-side anastomosis between the terminal ileum and ascending colon facilitated a larger passage for intestinal content, preventing anastomotic stenosis. We achieved surgery with a small pararectal incision and stoma on the abdominal wall, reducing the impact on aesthetic concerns and patients’ perception of their body image.
The adopted system for ABI guaranteed a complete bowel emptying in reasonable time (<30 min). Previous experiences reported 1–2 L of saline to complete the bowel washout. We individuated the exact amount of saline (about 600 ml) for a complete washout without any increased risks of bowel damages (i.e., perforation) thanks to our assisted training with the contrast medium under fluoroscopy. The periodical conduit catheterization limited the risk of stoma stenoses. A precise follow-up schedule evaluated the bowel management progressively, favored the adherence to treatment, detected minor problems at an early stage (i.e., sporadic episodes of FI/UTIs, difficulties with catheter insertion) and guaranteed a prompt treatment avoiding the occurrence of severe complications (e.g., stoma stenosis, bowel obstruction). We monitored our results through the NBD score.
All these issues represent a milestone of our MACE program, which enabled a complete bowel evacuation through ABI in all cases, avoiding colostomy.
This study represented the initial stage 2a of the IDEAL framework for surgical innovation [27]. Considering the reduced number of NBD patients requiring MACE, the most effective way to carry on the evaluation of our approach is through a multi-center prospective study to validate our workflow externally and perform extensive descriptive and inferential statistical analyses to identify risk factors for failures and subsets responding successfully.
To our knowledge, this is the first report of a multi-step approach for MACE, combining both a tapered terminal ileum conduit and a modified TAI system. Our initial experience was associated with encouraging results, in particular a decreased morbidity and a high success rate compared to other clinical series, so the authors advocate the adoption of similar programs by other centers to evaluate this technique use, especially considering the increasing prevalence and incidence of patients with NBD.
Data availability
The data collected and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Emmanuel A. Neurogenic bowel dysfunction. F1000Res. 2019;8:1–6.
Pires JM, Ferreira AM, Rocha F, Andrade LG, Campos I, Margalho P, et al. Assessment of neurogenic bowel dysfunction impact after spinal cord injury using the international Classification of Functioning, Disability and Health. Eur J Phys Rehabil Med. 2018;54:873–9.
Emmanuel AV, Krogh K, Bazzocchi G, Leroi AM, Bremers A, Leder D, et al. Consensus review of best practice of transanal irrigation in adults. Spinal Cord. 2013;51:732–8.
Christensen P, Kvitzau B, Krogh K, Buntzen S, Laurberg S. Neurogenic colorectal dysfunction - Use of new antegrade and retrograde colonic wash-out methods. Spinal Cord. 2000;38:255–61.
Weiser A, Stock J, Hanna M. Modified Cecal Flap Neoappendix for the Malone Antegrade Continence Enema Procedure: a Novel Technique. J Urol. 2003;169:2321–4.
Surfield GA, Andrews DA. Tapered terminal ileum conduit for antegrade continence enemas. Pediatr Surg Int. 2005;21:989–90.
Krogh K, Laurberg S. Malone antegrade continence enema for faecal incontinence and constipation in adults. Br J Surg. 1998;85:974–7.
Christensen P, Buntzen S, Krogh K, Laurberg S. Ileal neoappendicostomy for antegrade colonic irrigation. Br J Surg. 2001;88:1637–8.
Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–31.
Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. 2019;74:373–9.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo Classification of Surgical Complications. Ann Surg. 2009;250:187–96.
Spinelli M, Rizzato L, Renard J, Frediani L. A simple morpho-functional evaluation leads to a high transanal irrigation success rate in neurogenic bowel management. Pelviperineology. 2016;35:1–5.
Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J, et al. The Catz-Itzkovich SCIM: a revised version of the spinal cord independence measure. Disabil Rehabil. 2001;23:263–8.
Higgins P, Johanson J. Epidemiology of Constipation in North America: a Systematic Review. Am J Gastroenterol. 2004;99:750–9.
Christensen K, Doblhammer G, Rau R, Vaupel J. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208.
Schluter PJ, Askew DA, Jamieson HA, Arnold EP. Urinary and fecal incontinence are independently associated with falls risk among older women and men with complex needs: A national population study. Neurourol Urodyn. 2020;39:945–53.
Krogh K, Nielsen J, Djurhuus J, Mosdal C, Sabroe S, Laurberg S. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997;40:1233–9.
Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347:1651–3.
Stein DM, Knight WA. Emergency Neurological Life Support: Traumatic Spine Injury. Neurocrit Care. 2017;27:170–80.
Cotterill N, Madersbacher H, Wyndaele JJ, Apostolidis A, Drake MJ, Gajewski J, et al. Neurogenic bowel dysfunction: Clinical management recommendations of the Neurologic Incontinence Committee of the Fifth International Consultation on Incontinence 2013. Neurourol Urodyn. 2018;37:46–53.
Krogh K, Olsen N, Christensen P, Madsen JL, Laurberg S. Colorectal transport during defecation in patients with lesions of the sacral spinal cord. Neurogastroenterol Motil. 2003;15:25–31.
Emmanuel A, Kumar G, Christensen P, Mealing S, Størling ZM, Andersen F, et al. Long-term cost-effectiveness of transanal irrigation in patients with neurogenic bowel dysfunction. PLoS ONE. 2016;11:1–13.
Malone PS, Ransley PG, Kiely EM. Preliminary report: the antegrade continence enema. Lancet. 1990;336:1217–8.
Curry JI, Osborne A, Malone PSJ. The MACE procedure: Experience in the United Kingdom. J Pediatr Surg. 1999;34:338–40.
Gerharz EW, Vik V, Webb G, Leaver R, Shah PJ, Woodhouse CR. The Value of the MACE (Malone Antegrade Colonic Enema) Procedure in Adult Patients. J Am Coll Surg. 1997;185:544–7.
McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–12.
Acknowledgements
We thank our physiotherapist, Tatiana Bianconi, and the staff by our Neurourology Service for their help in managing patients and finding medical records.
Funding
The authors received no funding support to undertake this study.
Author information
Authors and Affiliations
Contributions
MS supervised the study. MS and GS contributed to study conceptualization, literature search, data analysis and interpretation. MS and GS wrote the original draft. LR, AS, FS and SC were responsible for collecting data and creating figures. EM contributed to evaluating study methods and revising the paper. OC contributed to project planning, review of data analysis, data interpretation, and paper revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Spinelli, M., Sampogna, G., Rizzato, L. et al. The Malone antegrade continence enema adapting a transanal irrigation system in patients with neurogenic bowel dysfunction. Spinal Cord Ser Cases 7, 34 (2021). https://doi.org/10.1038/s41394-021-00397-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00397-3