Abstract
The skeleton is a highly innervated organ in which nerve fibers interact with various skeletal cells. Peripheral nerve endings release neurogenic factors and sense skeletal signals, which mediate bone metabolism and skeletal pain. In recent years, bone tissue engineering has increasingly focused on the effects of the nervous system on bone regeneration. Simultaneous regeneration of bone and nerves through the use of materials or by the enhancement of endogenous neurogenic repair signals has been proven to promote functional bone regeneration. Additionally, emerging information on the mechanisms of skeletal interoception and the central nervous system regulation of bone homeostasis provide an opportunity for advancing biomaterials. However, comprehensive reviews of this topic are lacking. Therefore, this review provides an overview of the relationship between nerves and bone regeneration, focusing on tissue engineering applications. We discuss novel regulatory mechanisms and explore innovative approaches based on nerve–bone interactions for bone regeneration. Finally, the challenges and future prospects of this field are briefly discussed.
Similar content being viewed by others
Introduction
The repair and reconstruction of missing or dysfunctional organs remain major challenges in the field of biomedical science.1 In the context of bone tissue, for small bone defects, various cell types can be mobilized to initiate endogenous repair through synergistic mechanisms.2 However, when the size of the bone defect exceeds a critical threshold, spontaneous healing is insufficient, necessitating additional interventions. Over the past few decades, researchers have developed diverse bioactive materials to repair bone defects, encompassing bone-mimetic ceramics, metal-based scaffolds, and natural or synthetic polymers.3,4 Unfortunately, these biomaterials merely replicate the macroscopic structure and mechanical characteristics of bones, with a limited ability to imitate the functional units within bones.5 Nevertheless, the rejuvenation of vascular and neural elements within newly formed bone structures is crucial for achieving optimal bone healing.6 In recent studies, angiogenesis has emerged as a critical index for assessing the efficacy of bone repair materials.5,7 Conversely, despite the presence of nerves in various regions of bone tissue, such as the periosteum and bone marrow, they are often overlooked in the design of bone repair materials.1,8
In recent years, there has been a growing focus on skeletal regulation by nerves, leading to the emergence of innovative theories and concepts. The importance of nerves in the process of regeneration was initially noted in research involving amphibian amputations and subsequently became a widely recognized phenomenon in mammals. Hence, investigators have endeavored to elucidate the mechanisms governing neuronal communication of the central and peripheral nervous systems with cells in the skeletal microenvironment, delineating their contributions to the growth, remodeling, and repair of bone.8 Studies on embryological anatomy have revealed that neural tissue development precedes bone tissue development during embryogenesis. This order of events occurs not only because the embryonic brain forms before the skeletal system but also because of the sequential pattern of innervation in embryonic bone development, with initial innervation occurring in the central portion of the backbone and later extending to the metaphysis until bone canals form around the neural pathways.9
As an early event in the process of bone repair, neural growth occurs prior to bone regeneration.10 A lack of nerve growth factor (NGF) signaling leads to impaired sensory innervation, delayed vascularization, decreased osteoprogenitor cells, and hindered skeletal development.11,12 More specifically, peptidergic nerves can directly influence bone formation by releasing neurogenic factors. These factors, including calcitonin gene-related peptide (CGRP), substance P (SP), vasoactive intestinal peptide (VIP) and neuropeptide Y (NPY), participate in bone formation and bone metabolism by interacting with receptors on bone cells.13 Nonosseous systems, including the vascular system and immune system, are also crucial participants in bone regeneration. Blood vessels play a vital role in providing nutrients and oxygen during bone regeneration, while nerves actively regulate bone metabolism and guide angiogenesis through the secretion of neurotransmitters and neuropeptides.14 Patients with neuropathy, such as congenital insensitivity to pain (CIP), are more prone to skeletal complications such as bone infections, fractures, and joint dislocations.15 Additionally, changes in innervation and activation are associated with orthopedic diseases such as osteoarthritis, lower back pain, and osteoporosis.16 The intricate interplay between nerves and bone highlights the importance of neurogenic regulation in bone metabolism and healing.
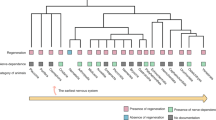
While previous reviews have touched upon the interplay between bone and the nervous system, there is a noticeable dearth of comprehensive investigations specifically examining the utilization of nerve-related components in the realm of bone tissue engineering. In this review, we first introduce the relationship between nerves and tissue regeneration and delve into the mechanisms by which nerves regulate bone regeneration. By examining the relationships between nerve regeneration, vascularization and the expression of neuropeptides following bone fracture, we emphasize the importance of the role of nerves in bone regeneration. Additionally, we introduce the concept of neuro-bone tissue engineering and present potential biomaterials for application in this approach. Finally, we propose six neuro-bone tissue engineering strategies aimed at advancing and translating the field and discuss their prospects. We expect this review to provide a reference for the study of nerve–bone crosstalk and to enrich the theoretical understanding of neuromodulatory osteogenesis, contributing to the development of bone tissue engineering and regenerative medicine (Fig. 1).
Nerve-mediated regulation of bone metabolism and neuro-bone tissue engineering research. a The peripheral nervous system and central nervous system regulate bone homeostasis and regeneration through neurogenic factors and neural circuits. b, c Neurogenic signals can act on various cells (osteoblasts, osteoclasts, macrophages, and endothelial cells) in bone tissue and generate bioactive responses (angiogenesis, neurogenesis, bone formation, and bone resorption), indicating their potential for use in bone tissue engineering. d Each component among the four elements (scaffolds, seed cells, noncell bioactive factors, external stimuli) of neuro-bone tissue engineering offers multiple choices. Created with BioRender.com
Role of nerves in tissue regeneration
The importance of nerves in the regeneration process was initially observed through studies involving the amputation of amphibian limbs. Many organisms, such as salamanders, starfish, crabs, and lizards, possess the remarkable ability to fully regenerate certain limbs.17,18 In fact, flatworms can even be divided into multiple pieces, each of which has the capacity to regenerate into a complete organism.19 These remarkable regenerative capabilities in various organisms have sparked great interest among researchers in the field of regenerative medicine. In particular, the role of nerves in this regenerative process has garnered significant attention. In a study involving salamanders, it was observed that denervation at the brachial plexus level hindered the regeneration of upper limbs.20 Conversely, redirecting nerves toward the site of injury showed promise in promoting limb regeneration and even the development of supernumerary limbs.21 Notably, a clear connection was observed between the degree of denervation and the hindrance of limb regeneration, indicating the essential involvement of peripheral nerves in the precise control of limb regrowth.22
Nerves are also essential for the regeneration of multiple tissues in vertebrates (Fig. 2). Since mouse toe tips exhibit impaired regeneration in the absence of local nerve innervation, nerves within bone are postulated to act as mediators of bone regeneration.23 Both sensory and sympathetic nerves are present in the callus and express tyrosine hydroxylase, CGRP, and SP as early markers before vascularization occurs.10 Nerve signals within bone can either accelerate or impede osteogenesis, maintaining the balance of bone metabolism. Peripheral nerves provide precise spatiotemporal coordination of this process during both physiological bone remodeling and repair. Furthermore, nerve-resident cells such as fibroblast-like mesenchymal cells and Schwann cells undergo transformation and proliferation upon nerve injury. Subsequently, they relocate to the injury site to facilitate bone regeneration in mice.13 Histomorphometry and immunohistochemical analyses showed that sensory innervation deprivation and CGRP inhibition can suppress bone remodeling and generate a proinflammatory environment.24 One investigation assessed how the inferior alveolar nerve affects new bone formation in rabbits and found that the absence of sensory nerves could lower the quality of newly formed bone during mandibular distraction osteogenesis.25 These observations suggest the involvement of peripheral nerves during bone regeneration.
The nervous system serves as the foundation of skin physiological functions and regulates wound healing and scar formation through multiple mechanisms. In clinical practice, skin wounds in patients with central nervous system injuries, such as those in the brain or spinal cord, have the potential to heal. However, chronic ulcers on the skin of diabetic patients, accompanied by peripheral neuropathy, present greater challenges for healing.26 Nerves actively promote skin wound healing through various mechanisms. These mechanisms include initiating neurogenic inflammatory responses, secreting neurotrophic factors to enhance blood supply to the tissues surrounding the wound, promoting the proliferation of fibroblasts and keratin-forming cells, and regulating the expression of type I and III collagen. Nerves also interact with the immune system and release neuropeptides.27,28,29 Additionally, exogenous neuropeptides show the potential to promote the healing of stubborn wounds. Conversely, denervation measures such as neuropeptide antagonists or highly selective neurotomy may help reduce scar growth.30,31
The restoration of liver tissue mass occurs through a combination of hepatocyte proliferation and hypertrophy in the remaining lobe after partial hepatectomy.32 The regenerative response to partial hepatectomy is negatively impacted by hepatic vagotomy, and the activity of the sympathetic nervous system may be suppressed during the initial stages of regeneration.33 In rats, hepatic vagotomy resulted in notably smaller liver-to-body weight ratios seven days after partial hepatectomy due to reduced hepatocyte proliferation.34 Performing hepatic vagotomy before partial hepatectomy increases mortality in mice, but this effect can be counteracted by the overexpression of FoxM1, a regulator of hepatocyte proliferation within the vagus-macrophage-hepatocyte signaling network.35 Sensory nerves also contribute to hepatocyte proliferation following partial hepatectomy. Neurons expressing CGRP innervate the biliary tree, and CGRP expression is heightened in rat livers after partial hepatectomy.36 RAMP1, a component of the CGRP receptor, is crucial for hepatocyte proliferation, as evidenced by significantly delayed regeneration in RAMP1 knockout mice following partial hepatectomy.37
Skeletal muscle, like other tissues, relies on vascularity and innervation for proper function. Peripheral nerves establish neuromuscular junctions (NMJs) to innervate local skeletal muscle tissue, which is essential for muscle survival, development, maturation, and contraction.38 When skeletal muscles are denervated, they lose contractility and undergo muscle atrophy.39,40 Neurotrophic factors and neurotransmitters released from neural components play a crucial role in increasing skeletal muscle cell survival and differentiation.38,41 Furthermore, nerves promote the regeneration of muscle blood vessels, ensuring an adequate supply of oxygen and nutrients to support muscle regeneration and repair.42,43
The role of nerves in tissue repair has been studied in both mammals and phylogenetically distant animals. Studies have shown that nerves regulate regeneration through the secretion of neuropeptides and neurotransmitters. Understanding these mechanisms can help identify therapeutic approaches for tissue regeneration.
Neuromodulation of bone
Central nervous system and bone
The brain and spinal cord constitute the central nervous system, which analyzes and integrates information and generates coordinated responses to stimuli44 (Fig. 3). The cell bodies of afferent sensory nerves are typically located in the dorsal spinal root ganglion, which connects to the central nervous system.1 Additionally, pseudorabies virus was detected in the sacral intermediolateral cell column and the central autonomic nucleus after inoculation into the metaphysis of the distal femur, providing evidence for the connection between autonomic nerves within bones and the central nervous system.45,46
Peripheral nerves in the lower limbs originate or terminate in the lumbar spinal cord and sacral spinal cord. Both the sympathetic (blue) and parasympathetic (green) systems use two neuron relay systems to communicate with peripheral targets. The sympathetic innervation of the lower extremities originates from the lumbar spinal cord, while the parasympathetic innervation comes from the sacral spinal cord. The primary sensory neurons (red) are pseudounipolar neurons whose cell bodies are located in the dorsal root ganglion. Created by BioRender.com
An interesting phenomenon is that patients with fractures and concomitant traumatic brain injuries show increased callus formation and faster fracture healing. This suggests that brain injury may affect multiple factors during the healing process, including the release of inflammatory factors, serum leptin concentration, and the differentiation ability of mesenchymal stem cells.47 Injury to the spinal cord or central nervous system raises the risk of heterotopic ossification, particularly in patients who have recently undergone total hip replacement or have paraplegia.48,49 Additionally, spinal cord injuries caused by overstretching, trauma, or compression can result in a decrease in bone mineral density, which may be related to the upregulation of the WNT signal.50,51
There is a prevailing belief that skeletal system metabolism is intertwined with the endocrine system.52 Nevertheless, the revelation of the regulatory influence exerted by numerous neural circuits and neuropeptides has significantly increased our awareness of the involvement of the central nervous system in governing bone metabolism.53,54 We will describe this relationship in the following sections. Given our review’s primary focus on exploring the application of central nervous system regulation in bone tissue engineering, we advise readers to refer to earlier reviews for a broader understanding of the central nervous system’s effects on bone tissue.53,55,56,57
Serotonin
Throughout key brain regions, serotonin (5-HT) plays a crucial role as a neurotransmitter in learning, memory, and attention.58 The discovery of the presence of 5-HT receptors in major bone cell types, such as osteoblasts, osteocytes, and osteoclasts, has sparked significant interest in understanding its involvement in the regulation of bone metabolism.
In recent years, several investigations have substantiated the adverse impact of peripheral 5-HT on bone formation, specifically its role in promoting bone resorption while inhibiting bone formation.59,60,61 Yadav et al. discovered elevated serotonin levels in osteoporosis-pseudoglioma syndrome patients, which may be linked to osteoporosis symptoms. Animal studies confirmed that increased peripheral 5-HT leads to lower bone density and structural damage. Additionally, peripheral 5-HT slows the proliferation and differentiation of mouse osteoblasts, suggesting that it directly inhibits bone formation and promotes resorption via bone tissue receptors.62,63
In contrast to the effects of peripheral 5-HT, the central nervous system’s regulation of bone tissue by 5-HT is associated with leptin and the sympathetic nervous system. Central 5-HT can suppress sympathetic activity, thereby indirectly inhibiting bone resorption and stimulating bone formation.64 Conversely, leptin can inhibit central 5-HT, enhance sympathetic activity, and exert a negative influence on bone formation. Furthermore, epidemiological data show that selective serotonin reuptake inhibitors (SSRIs), which inhibit 5-HT reuptake, can reduce bone density and raise fracture risk.65,66
Semaphorin 3 A
Semaphorin 3 A (Sema 3 A), a member of the semaphorin family, is highly expressed in the hypothalamus and functions as an axon-guiding molecule, contributing to the proper development of the nervous system.67 Its receptor, neuropilin-1 (Nrp-1), is widely expressed in bone tissue, suggesting that Sema3A plays a role in connecting nerves and bone.
It has been demonstrated that the interaction between Sema3A and Nrp-1 can activate the Wnt/β-catenin signaling pathway in osteoblasts, inhibiting adipogenesis and suppressing the osteoclastogenesis induced by receptor activator of nuclear factor-κB ligand (RANKL). This inhibition occurs through suppression of the immunoreceptor tyrosine-based activation motif (ITAM) and RhoA signaling pathway.68 Studies conducted on Sema3A- and Nrp-1-deficient mice, which exhibited an osteoporotic phenotype, showed a decrease in osteoblasts and impaired bone formation, indicating that Sema3A promotes osteoblast differentiation.69 Similarly, mice receiving intravenous injections of Sema3A demonstrated increased bone accumulation and faster bone regeneration.68 Furthermore, according to Fukuda et al.,70 neurospecific Sema 3A-deficient mice were similar to global Sema3A knockout mice, showing a markedly low bone mass phenotype, which revealed the association between observed skeletal abnormalities and neuron-derived Sema3A.71 These studies suggest that Sema3A holds promise as an osteoprotective agent.
Brain-derived neurotrophic factor
Brain-derived neurotrophic factor (BDNF) is a versatile protein that is crucial for neural system development and is localized within a subset of dorsal root ganglia (DRG) neurons.72 Peripheral inflammation triggers an increase in BDNF mRNA and protein levels in TrkA+ sensory neurons. Subsequently, BDNF is transported to the spinal cord, where it facilitates central sensitization to pain.73
Both BDNF and its receptor TrkB have been identified in fracture tissues during inflammation and early bone formation, primarily in endothelial and osteoblastic cells.72 BDNF was found specifically in granulation tissue at the margins of woven bone and was absent in chondrocytes and mature bone.72,74 Osteocytes displayed strong TrkB expression, and in an in vivo study with cortical osteotomy, BDNF was observed to boost osteosclerosis. This finding implies that osteocytes may influence bone healing by regulating BDNF.75 BDNF has been verified to promote the proliferation and differentiation of human bone marrow mesenchymal stem cells (hBMSCs) by elevating integrin β1 expression through TrkB-mediated activation of the ERK1/2 and AKT signaling pathways.76,77 BDNF additionally amplifies RANKL production by hBMSCs, thereby facilitating osteoclastogenesis.78 These apparently contradictory impacts on bone may arise from the epigenetic control of BDNF transcription, wherein distinct physiological or pathological circumstances lead to alternative splicing and polyadenylation.13
Peripheral nervous system and bone
The peripheral nerves that innervate human bones consist primarily of sensory and motor nerves, which transmit stimuli or responses through action potentials or chemical signals. These innervations are distributed throughout the periosteum, trabeculae, subchondral bone, and bone marrow79 (Fig. 4). Motor nerves within the bone mainly consist of autonomic nerves, which are classified as sympathetic or parasympathetic based on their pharmacological characteristics. However, the specific density and pattern of parasympathetic nerves in the bone remain a topic of debate.80
Sensory nerve innervation is crucial for bone remodeling and repair.6 Damage to sensory innervation slows bone formation and increases bone resorption, resulting in reduced trabecular thickness and increased bone fragility.81 In a rat-angled fracture model, there was a higher presence of CGRP-positive innervation in the angulated concave regions than in convex regions, suggesting a greater need for sensory innervation in the concave region for bone repair.82 Numerous sprouted nerve fibers could be observed in the hyperplastic periosteum, callus, and fibrocartilage edge of a tibia fracture before vascular ingrowth and bone mineralization.10,83 In addition to its role in regulating the internal environment, the autonomic nervous system maintains bone homeostasis.57 Studies have shown that sympathectomized rats exhibit significantly reduced mineral content and density in the lumbar vertebrae.84 Mice lacking β2-adrenergic receptor expression still displayed a high bone mass phenotype after ovariectomy.85 Additionally, after ovariectomy, nerve density in rats decreased significantly, supporting the connection between the nervous system and bone loss following ovariectomy.86 Furthermore, microgravity and inner ear vestibular dysfunction-induced bone loss appear to be associated with sympathetic overactivity.87,88
Neurogenic factors, including CGRP, SP, VIP, and NPY, as well as neurotransmitters such as norepinephrine (NE) and acetylcholine (Ach), play a crucial role in the influence of peripheral nerves on bone.80 These factors bind to specific receptors present in bone tissue, affecting processes such as bone formation, bone resorption, angiogenesis, and macrophage polarization. These effects have important implications for bone homeostasis and bone regeneration. In the subsequent sections, we will provide a detailed description of these mechanisms.
Calcitonin gene-related peptide
CGRP is synthesized primarily in the DRGs and transported to synaptic vesicles to regulate the activity of bone cells.89 Within the bone, CGRP-positive nerve fibers are more densely concentrated in the periosteum, which is crucial for new bone formation, than in the trabeculae and bone marrow.90
Research has shown that CGRP upregulates osteogenesis-related genes and promotes the mineralization of osteogenic cells, both dose-dependent effects.91,92 By binding to the RAMP1-CLR complex, CGRP released from sensory nerve endings stimulates osteogenic differentiation in periosteum-derived stem cells through the cAMP-CREB pathway.90 Further research has substantiated that CGRP inhibits bone resorption by increasing the OPG/RANKL expression ratio in osteoblasts. Likewise, CGRP induces the intracellular accumulation of cAMP, promotes insulin-like growth Factor I (IGF-I) expression, and hinders the production of tumor necrosis factor-alpha (TNF-α) in osteoblasts, thereby promoting a favorable bone metabolic balance.93 Additionally, it inhibits osteoclast differentiation by suppressing the RANKL-NF-κB signaling pathway.94,95
Through its interaction with inflammatory factors, CGRP can augment vascular permeability and facilitate vasodilation, consequently exerting regulatory control over blood flow within blood vessels.96 Additionally, CGRP released from DRGs is associated with angiogenesis during osteoporotic fracture healing and critical bone defect regeneration, which greatly affects nutrient supply during fracture healing.97,98
Moreover, CGRP has emerged as a potent immunomodulatory factor that can interact with macrophages to promote their transformation from M1 to M2 subtypes, thereby inhibiting inflammation in local tissues and promoting tissue healing.99,100 Notably, this may be related to the PI3K/AKT signaling pathway and necessarily involves the assistance of other cytokines.101,102
Substance P
Similarly, SP is a neuropeptide that is widely distributed throughout the nervous system.103 SP-positive nerve fibers are found predominantly in regions of the skeletal system with high metabolic activity, including long bones, periosteum, joints, and epiphyseal growth plates.104
The impact of SP on bone remains contentious, possibly due to variations in its concentration. Recent studies have shown that high concentrations of SP (>10−8 mol·L−1) can upregulate osteogenic genes in bone marrow-derived mesenchymal stem cells (BMSCs), potentially by modulating autophagic activity and reactive oxygen species generation.105,106 In contrast, low concentrations of SP (<10−8 mol·L−1) inhibit the osteogenic differentiation of BMSCs and only maintain their self-proliferation.107 Additionally, it has been reported that SP can enhance endogenous mesenchymal stem cell recruitment during calvarial bone defect repair.108 On the other hand, SP has been implicated in enhanced bone resorption through the RANKL/OPG axis.109,110 The activation of its receptor, NK1-R, and downstream NF-κB signaling pathways may contribute to increased osteoclastogenesis.111,112 Noticeably, either excessive release or depletion of SP has been associated with increased numbers of osteoclasts, as observed in capsaicin-induced inactivation of sensory neurons with reduced SP expression, which leads to an osteoporosis phenotype.113 Hence, SP simultaneously regulates the functions of osteoblasts and osteoclasts in a dose-dependent manner.
In addition, SP has been shown to have a variety of biological functions, including angiogenesis and immune regulation. The inhibition of endogenous endopeptidase activity or exogenous SP injection has been shown to accelerate angiogenesis during wound healing, which is associated with enhancing granulocyte recruitment and activation.114,115,116 Spinal cord injury functional recovery after SP injection may be associated with the M2 polarization of macrophages.51,110,117
Neuropeptide Y
NPY, a prominent neuropeptide, is predominantly synthesized and expressed within the nervous system.118 In the peripheral nervous system, NPY coexists with NE in the sympathetic nerves.119 NPY is involved in a range of biological functions, including vasoconstriction, the regulation of appetite and energy balance.107 Notably, NPY also plays a pivotal role in bone metabolism and the regulation of remodeling. These effects are mediated through direct and indirect signaling via Y1 and Y2 receptors, respectively.120
However, the reported bone formation outcomes induced by NPY include varying and inconclusive findings. Previous studies have demonstrated that mice with genetic knockout of NPY exhibited elevated bone density, increased mineral content, and enhanced cortical bone formation.121 Additionally, the induction of adipogenesis by NPY and its inhibitory effect on BMSC osteogenesis have been linked to the aging process and are closely connected with the development of osteoporosis.122 During fracture repair, heightened NPY-positive innervation was detected on the convex side of tibial angled fractures, hinting at a possible association with diminished callus thickness on the same side.123 In contrast, NPY was found to promote the proliferation and differentiation of bone marrow stromal cells into osteoblasts through the activation of the Wnt/β-catenin pathway in rat experiments.124 Furthermore, NPY mitigates excessive stress-induced bone loss by modulating central and peripheral noradrenergic neurons through Y2 receptor mediation.125 NPY has also been shown to promote postfracture bone healing through Y1 receptor signaling.126 Additionally, global deletion of the Y1R gene hampers the initial stages of fracture repair, resulting in reduced callus volume and strength, consequently leading to delayed fracture healing.127
Notably, NPY also influences the expression of VEGF as well as the migration of BMSCs in vitro, suggesting that it may be involved in angiogenesis during fracture healing.128 Therefore, further investigation is warranted to better comprehend the intricate effects of NPY on bone homeostasis and healing.
Vasoactive intestinal peptide
VIP is highly prevalent in the periosteum, and nerve fibers containing VIP are broadly distributed throughout the Haversian system and within Volkmann’s canal, which extends from the diaphysis to the epiphysis.129 Generally, VIP coreleases alongside ACh from cholinergic nerve terminals and exerts its physiological effects by activating VPAC1 and VPAC2 receptors.130 VIP has emerged as a multifunctional molecule with diverse physiological functions, including its role in bone tissue homeostasis and regeneration.129
In vitro investigations have revealed that VIP may augment the osteogenic differentiation of BMSCs through activation of the Wnt/β-catenin signaling pathway.131 Moreover, a reduction in VIP levels within the bone has been noted following ovariectomy, and this decrease is associated with the development of postmenopausal osteoporosis.132 Further research has demonstrated that VIP can enhance osteogenic marker expression and promote bone fracture healing in mice subjected to sympathectomy.133 Additionally, a recent study showed that VIP can inhibit osteoclast differentiation and temporarily suppress osteoclast activity and bone resorption.134
Unlike some other neuropeptides, VIP exhibits notable biological activity in blood vessels, making its utilization in bone repair a promising prospect. VIP plays a role in angiogenesis in a variety of conditions, such as arthritis and tumors.135,136 The gradual release of VIP within the wound has a positive impact on granulation tissue growth and angiogenesis, leading to a substantial enhancement of the wound healing process.137 Furthermore, because VIP has a vasodilatory effect on blood vessels, it augments local tissue blood flow, ensuring an ample supply of nutrients that are crucial for wound healing.138 Finally, a recent study found that VIP stimulates tubular formation in endothelial cells (ECs) and enhances the expression of VEGF in BMSCs.129
Norepinephrine and acetylcholine
NE serves as the pivotal neurotransmitter in the sympathetic nervous system and exerts its function mainly through the widely expressed β‐adrenergic receptors (β‐AR) in bone tissue.139,140 Elevated sympathetic activity is well known to increase epinephrine levels in urine, consequently suppressing osteogenic activity. Specifically, NE inhibits hBMSC proliferation by activating β2-AR and inducing ERK1/2 and PKA phosphorylation.141 The impact of NE on BMSCs may contribute to impaired bone formation, possibly explaining the increased bone loss observed with glucocorticoid use.141,142 Additionally, NE stimulates osteocytes to produce RANKL, a crucial factor in osteoclast differentiation, ultimately enhancing osteoclast maturation.143 NE also directly boosts osteoclastogenesis by mediating intracellular ROS production, and the use of β-AR inhibitors such as propranolol has demonstrated corresponding inhibitory effects on this process.144,145 Importantly, the administration of propranolol to mice with femur fractures and posttraumatic stress disorder (PTSD) ameliorated the observed impairment of new bone formation.146 These research findings suggest that countering the influence of norepinephrine on bone tissue has potential as an approach for bolstering bone regeneration.
Likewise, ACh acts as the principal neurotransmitter released by cholinergic nerve fibers and exerts its biological functions by binding to nicotinic acetylcholine receptors (nAChRs) or muscarinic acetylcholine receptors (mAChRs) on cells.147,148 Prior studies have demonstrated that ACh stimulates osteoblast proliferation but has a limited effect on osteoblast differentiation.45 Ligands targeting nAChRs can dose-dependently decrease the count of osteoclasts and tartrate-resistant acid phosphatase-positive monocytes in vitro.149 Similarly, mice lacking the α(2)nAChR subunit exhibit elevated bone resorption and reduced bone mass.45 The activation of nAChR inhibits RANKL-induced Ca2+ oscillation, negatively regulating the osteoclastogenesis process through weakened Ca2+-NFATc1 signaling.149,150 Moreover, research results have indicated on the importance of cholinergic mechanisms during skeletal embryonic development. Experiments involving the implantation of ACh-soaked beads into chicken limb anlagen resulted in in ovo accelerated bone formation.151,152 Notably, it is crucial to clarify the distinct roles of mAChR subtypes within the skeletal system. A comprehensive understanding of the in vivo biological effects of ACh on bone tissue remains elusive, highlighting the need for future research.
Skeletal interoception
Although it is generally believed that the peripheral nervous system and the central nervous system have independent effects on the skeletal system, recent research has revealed a coordinated mechanism indicating that they may jointly participate in skeletal interoception.153 In this mechanism, PGE2, a member of the prostaglandin family, plays a key role by binding to EP4 receptors. Essential enzymes in the biosynthesis of PGE2, include prostaglandin E2 synthase-1 (mPGES-1) and cyclooxygenase (COX).154 Interestingly, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), which are COX enzyme inhibitors, for pain management may trigger adverse effects such as delayed fracture healing and increased risk of nonunion. This effect further supports the idea that the disruption of PGE2/EP4 signaling may hinder bone regeneration.155 Although PGE2 is not directly released by neurons, it can activate skeletal interoception and initiate the regulation of bone tissue metabolism and regeneration by nerves, providing a new perspective on nerve–bone interactions.
Research on skeletal interoception strives to enhance our comprehension of how skeletal sensory nerves sense the state of bone tissue. The prevailing belief is that ascending signals from bone tissue reach the central nervous system, particularly the hypothalamus, via sensory nerves, dorsal root ganglia, and the superficial dorsal horn of the spinal cord (Fig. 5).153 Reportedly, PGE2 from osteoblasts during bone resorption can bind to EP4 receptors on sensory nerves as an ascending signal that can sense bone density and thereby modulate autonomic nerve activity via the hypothalamus.144 Reducing sympathetic tone through these neural circuits promotes mesenchymal stem cell transformation into osteoblasts and inhibits local adipogenic effects, which is crucial for maintaining bone mass.156 In aging-related disc degeneration, vertebral endplates become porous. Low-dose celecoxib, a COX-2 inhibitor, can maintain PGE2 levels, mitigating endplate porosity and pain via inflammation and skeletal interoception regulation.157 Certain metal ions from implants, such as Mg2+, Zn2+, and Cu2+, stimulate PGE2 secretion by macrophages, aiding bone regeneration through this neural circuit and revealing the role of divalent cations in healing.158 This discovery not only underscores the pivotal role of implants in bone repair but also emphasizes the importance of specific metal ions in orchestrating the intricate interplay between nerves and bone. It opens up new avenues for the development of more efficient implant materials and treatment strategies, exhibiting potential for positively influencing bone health and the healing process. The details of crosstalk between the brain and skeletal tissue remains a mystery.159 However, this interaction is vital for overall bodily equilibrium. Delving into these mechanisms is crucial, as it has the potential to yield tangible clinical advancements.
Skeletal interoception regulates bone metabolism by coordinating sensory and sympathetic nerve activity. Bone implants containing Mg2+, Zn2+, and Cu2+ can enhance bone regeneration by activating skeletal interoception. Activated skeletal interoception participates in maintaining bone homeostasis by regulating stem cell differentiation lineage. In bone degenerative conditions, modulating the concentration of PGE2 by celecoxib to maintain a physiological level can activate skeletal interoception, which contributes to decreased endplate porosity and to pain relief. Created with BioRender.com
Activity of nerves during bone fracture and regeneration
During the process of bone regeneration, nerves play an active role in responding to damage signals caused by external forces or pathological changes that disrupt the bone structure. These injury signals propagate in a retrograde direction along the proximal axon to the cell bodies, triggering the transition of nerves into a regenerative state. This transition involves the initiation of regeneration-related metabolism and the secretion of neuropeptides such as CGRP and SP around the fracture site.97,98,99 The terminals of sensory nerves are equipped with various receptors, including Nav1.7, Nav1.8, Nav1.9, TRPV1, and TRPA1, which can be activated by inflammatory mediators and growth factors. The activation of these receptors leads to sensitization and the release of additional neurotransmitters.12,160,161 Concurrently, the distal axon undergoes Wallerian degeneration, resulting in deterioration of the axon, myelin, and blood-nerve barrier. Schwann cells (SCs) play a role in clearing debris that contains signals impeding axonal growth, and recruited macrophages contribute to the clearance process at the site of injury.162 Repair SCs generate provisional channels called SC basal lamina tubes, which serve as guides for regenerating proximal axons, directing them toward the target organ for reinnervation of the bone.163 In summary, the primary reactions of peripheral nerves to bone fractures involve inflammation and Wallerian degeneration.
Bone regeneration is a complex process that involves the restoration of the bone’s original microarchitecture and simultaneous reinnervation. Although these processes may seem unrelated, they are intricately intertwined, as they occur concurrently during the regenerative process.6,13 Researchers have found that adequate innervation density is crucial for salamander regeneration, which suggests that hyperinnervation may be necessary after bone fractures.22 As the callus forms and matures, the regenerated nerves undergo sprouting as the bone matrix is deposited. Eventually, these nerves become restricted to the outer fibrous capsule of the hard callus after being trimmed. Reinnervation is facilitated by both CGRP-positive and TH-positive nerve fibers, with CGRP nerve fibers playing a primary role.10 After the completion of bone repair, the nerve fibers within the bone typically return to their normal levels. However, in cases of fracture nonunion, persistent hyperinnervation can be observed in the vicinity of the bone, periosteum, cortical bone, and bone marrow.164 If excessive innervation is not corrected promptly, it can lead to chronic pain-related pathological conditions. Following bone injury, various neuropeptides and neurotransmitters are expressed in the healing microenvironment, which mediates nerve regeneration and neuromodulation of bone regeneration.10,97,98 Notably, NGF derived from macrophages plays a critical role in skull bone regeneration by stimulating the inward growth of sensory nerves.10 The distribution of increased neuropeptides varies during the four classical phases of bone healing, namely, the inflammatory phase, callus formation, bone callus formation, and the remodeling phase10,13,165,166,167,168,169,170 (Fig. 6). The bioactive molecules generated during bone regeneration influence not only the peripheral nervous system but also the vasculature and immune system, thereby promoting bone regeneration processes.97,98,128,161,171
Expression of neuropeptides and related events during bone healing. The upper line chart displays the temporal sequence of events following a bone fracture. The x-axis represents the number of days after fracture, while the y-axis depicts the relative activity of different events. Neuropeptides (NGF, BDNF, CGRP, SP, NPY, and Sema3A) show a distinct distribution across the four phases (inflammatory, soft/cartilaginous callus, hard/bony callus, and remodeling) of bone healing. Created with BioRender.com
Neuro-bone tissue engineering
While bone tissue does exhibit a natural regenerative ability that is adequate for the repair of small areas of damage, large defects resulting from traumatic injuries, degenerative diseases, congenital anomalies, or surgical tumor removal necessitate medical intervention to achieve functional restoration.4 At present, autologous or allogeneic bone grafts are considered the gold standard for repairing bone defects.172 However, the use of autologous bone grafts requires additional surgery at the tissue acquisition site.173 Compared to autologous bone grafts, allogeneic bone grafts are rendered nonviable through irradiation or freeze-drying, which reduces their bone inductivity and may ultimately lead to graft failure.174,175 While cellular bone matrices have been recently developed to enhance the bioactivity of grafts, they carry an increased risk of immune rejection and do not guarantee cell viability during preservation and implantation.175 At the same time, allogeneic transplantation carries inherent risks related to disease transmission, reduced structural integrity, and higher costs.175 Driven by pressing clinical needs, the field of bone tissue engineering has emerged and undergone rapid development in recent decades.
The bone tissue engineering research field seeks to develop materials that exceed the capabilities of bone autografts and allografts.4 Currently, various types of materials are available for bone tissue engineering, including natural or synthetic polymers and inorganic materials.176,177,178,179 Some of these biomaterials successfully mimic the structure and mechanical properties of bone. Additionally, some researchers have used a layer-by-layer design to mimic the hierarchical microstructure of bone, and such strategies have achieved some success in bone defect repair. However, few approaches have been able to mimic the functional units within bone, such as blood vessels or nerves, which has led to suboptimal outcomes.54
As previously mentioned, nerve fibers are widely present in bone and, to some extent, influence bone tissue regeneration, a function that has gained widespread attention in recent years. Previous research has emphasized the close interaction between bone tissue and neural tissue in various pathophysiological states and proposed potential scaffolding strategies for neuro-bone tissue engineering.54,79,80 In the field of neuro-bone tissue engineering, scaffolds have been ingeniously engineered to carry neural cells or growth factors. This innovative approach has facilitated the development of intricate neural networks within the scaffolds, enabling nerve-coordinated bone regeneration. Furthermore, given the influence of neuropeptides on the bone regeneration process, direct loading of these neurogenic factors or harnessing the favorable effects of the nervous system on bone regeneration is also considered a viable strategy. In this process, the nerves provide support for angiogenesis within the scaffold. Simultaneously, the intricately coordinated interaction between neuronal elements and vascular formation accelerates bone cell growth and yields outstanding regenerative outcomes.5,8,54 Therefore, in contrast to singular approaches to bone tissue engineering or neural tissue engineering, neuro-bone tissue engineering emphasizes the synergistic role of nerves during the bone regeneration process. New evidence suggests that neuro-bone tissue engineering has broad application prospects, but its current application status and design concepts require further elucidation, which we will discuss in the following sections.
Scaffolds in neuro-bone tissue engineering
In bone defect repair, biomaterials serve as three-dimensional frameworks for cell attachment, growth, and differentiation. In neuro-bone tissue engineering, the scaffold material plays a crucial role in creating an environment conducive to the regeneration of both nerve and bone tissues and in facilitating communication between the two. While there are already various materials available for repairing neural and bone tissues separately, limited research has focused on constructing materials specifically for neuro-bone tissue engineering. However, it is logical to consider that materials with applications in repairing both types of tissues have potential for use in neuro-bone tissue engineering. Additionally, some materials promote bone regeneration by influencing neural activity and can be incorporated into neuro-bone tissue engineering scaffold materials. In the following sections, we will discuss these materials in more detail.
Polymers
Polymers are organic materials composed of atoms interconnected through covalent bonds. Both naturally derived and synthetic polymers are valuable in bone tissue engineering. Naturally derived polymers, such as collagen, gelatin, hyaluronic acid, chitosan, alginate, and silk, are produced by living organisms.5,180,181,182 They undergo degradation into carbon dioxide and water, resulting in minimal inflammatory reactions in vivo. Synthetic polymers, including polylactic acid (PLA), polyglycolic acid (PGA), and poly(lactic-co-glycolic acid) (PLGA), can degrade under physiological conditions but may release acids that induce inflammation.5,183,184 However, synthetic polymers offer versatility through chemical modifications and molecular alterations, allowing the customization of properties for specific applications, such as the incorporation of functional motifs to enhance cell-scaffold interactions.185 Table 1 summarizes examples of the applications of different materials in bone and nerve regeneration.
Inorganic materials
In bone tissue engineering, inorganic materials play a significant role, particularly bioactive ceramics and metals (Table 1). Bioactive ceramics such as hydroxyapatite and calcium triphosphate possess excellent biocompatibility and osteoconductivity due to their similar composition to bone. They also provide a continuous supply of phosphorus and calcium ions, which promote biomineralization. However, pure ceramic materials alone cannot recruit cells, regulate immunity, or promote neurovascular regeneration. Hence, they are often used in combination with other bioactive materials to better fulfill the needs of neuro-bone tissue engineering.186,187,188,189,190,191 Metals, such as titanium and stainless steel, are commonly used in clinical metal implants. They exhibit favorable biocompatibility and mechanical properties. However, the non-biodegradable nature of these implants often necessitates a second surgery for removal. On the other hand, magnesium, which has an excellent Young’s modulus similar to that of natural cortical bone, is an important metal in bone tissue engineering. Mg-based implants not only avoid stress shielding but also contribute to immune regulation, nerve regeneration, angiogenesis, and bone repair. Notably, studies have demonstrated that magnesium can stimulate the secretion of CGRP by nerves, effectively mobilizing endogenous signals for nerve–bone repair.97,191,192 Consequently, magnesium holds great potential for applications in neuro-bone tissue engineering.
Composite materials
In neuro-bone tissue engineering, the demand for composite materials arises from a multitude of intricate factors, making the use of a single material unfeasible and thus necessitating the utilization of composites of existing materials (Table 1). First, in the context of neuro-bone tissue engineering, the consideration extends beyond the mere repair and regeneration of bone tissue to encompass the crucial promotion of neural tissue regeneration. The regeneration of these two distinct tissue types has disparate biological and mechanical prerequisites. For instance, materials must offer mechanical support for bone tissue regeneration and create a conducive environment for the growth of neural cells. Composite biomaterials offer a promising solution to meet these diverse needs. Moreover, neuro-bone tissue engineering aspires to faithfully replicate the intricate structure and functionality found in natural biological tissues, which entails scaffolds that mimic the complex structures of both bone and neural tissues while delivering essential biological functionalities. Furthermore, the successful regeneration of bone hinges on the synergistic interplay between neural and bone tissues. The advantage of composite materials resides in their capacity to facilitate favorable interactions between neural and bone tissues through the precise modulation of material combinations, thereby fostering synergistic regeneration. Last, the utilization of composite materials serves as an effective strategy to surmount the limitations inherently associated with single materials.
Polymers offer excellent biocompatibility, as natural polymers such as collagen are inherent components of biological tissues, providing innate bioinformatic guidance that enhances cell adhesion and tissue regeneration. Furthermore, synthetic polymers can be purposefully tailored, aligning them with desired biomaterial functions without altering their intrinsic properties.193 However, polymers may have relatively weak mechanical properties that do not necessarily suffice for the high strength demands of neuro-bone tissue engineering.4 In contrast, inorganic materials such as bioceramics and metals exhibit outstanding mechanical properties suitable for bone repair. They typically do not elicit immune responses and are thus well tolerated in the body.4 Nevertheless, some inorganic materials may lack the bioinformatics guidance needed for promoting cell adhesion and tissue regeneration, necessitating additional enhancement efforts.194,195 Furthermore, certain inorganic materials may induce adverse reactions in biological systems, necessitating careful screening and design.196
Considering these factors comprehensively, the common practice is to employ a composite materials approach by integrating polymers and inorganic materials to maximize the exploitation of their respective advantages while simultaneously mitigating their individual limitations. This integrated utilization can lead to more comprehensive performance and biocompatibility, offering the prospect of achieving significant breakthroughs in the field of neuro-bone tissue engineering. Thus, polymers and inorganic materials play complementary roles in biomedical engineering, offering increased hope and potential for tissue treatment and repair.
Seed cells in neuro-bone tissue engineering
Seed cells are fundamental elements in tissue engineering. Ideal seed cells for bone tissue engineering should possess strong proliferative capacity, robust adaptability to the environment, and good tissue compatibility.175 As seed cells are the primary contributors to the biological activity of bone tissue engineering scaffolds, previous research in bone tissue engineering has primarily emphasized their osteogenic differentiation potential, often overlooking the influence of seed cells on other endogenous cells. While all types of stem cells can undergo secondary differentiation, inducing stem cells into neuronal and osteogenic lineages simultaneously is undeniably challenging. From another perspective, in the field of neuro-bone tissue engineering, all seed cells that can promote the synergistic regeneration of neural and bone tissues should be considered. To date, various seed cells, including mesenchymal stem cells, Schwann cells, and endothelial cells, have been employed in neuro-bone tissue engineering.
Bone marrow mesenchymal stem cells
BMSCs are a subpopulation of cells found in the mammalian bone marrow stroma. They have remarkable ability to differentiate into various cell types, including bone, cartilage, adipose, neural, and myogenic cells, making them ideal seed cells for neuro-bone tissue engineering.197 In theory, any stem cell capable of differentiating into either bone or nerve cells can be used, but it is challenging to simultaneously induce neural and osteogenic differentiation in the same stem cells. Therefore, BMSCs are often coimplanted with other cells, particularly SCs. Coculturing SCs and osteoblasts has been found to maintain the normal secretion of neurotrophic factors by SCs and to promote the proliferation and differentiation of osteoblasts.198 In a recent study, a bilayered structure was constructed, mimicking the spatial distribution of cranial bone and skull-associated nerves. This biomimetic design greatly facilitated bone regeneration by strategically distributing SCs and BMSCs.199 Additionally, combining sensory nerves with BMSCs on β-TCP scaffolds and implanting them into critical defect sites in rabbit femurs enhanced neovascularization and new bone formation.200 Studies have also shown that the BMSC-derived extracellular matrix, which contains various microenvironmental cues, such as biochemical, spatial, and biomechanical factors, provides a favorable environment for nerve regeneration.201 When mesenchymal stem cell-conditioned medium was loaded onto a 3D-polycaprolactone (PCL) scaffold, it effectively promoted nerve regeneration after axotomy.202 Overall, the regenerative effects of BMSCs are attributed to their ability to differentiate and replace damaged tissues, as well as their interaction with surrounding tissues through paracrine signaling. This creates a suitable microenvironment that jointly regulates the proliferation and differentiation processes of tissues and organs. However, achieving the codelivery of multiple seed cells in a scaffold still requires compromises in the crosstalk and interactions between the different cell types.198
Schwann cells
Schwann cells are peripheral glial cells that play a crucial role in peripheral nerve regeneration. Following nerve injury, SCs collaborate with macrophages to clear myelin debris and proliferate to form bands that support axon growth by secreting growth factors and extracellular matrix.203 The transplantation of SCs has been shown to enhance axon outgrowth both in vitro and in vivo.204,205 Moreover, SCs have been found to exert nutritional and regulatory effects on bone metabolism through complex mechanisms. In a mouse mandibular osteotomy model, the absence of SCs and their paracrine factors resulted in functional defects in mandibular skeletal stem cells, leading to a decreased rate of mandibular bone repair.206 These findings highlight the importance of SCs and their paracrine effects in the regenerative differentiation of skeletal stem cells. Recent studies have also demonstrated that Schwann cell-derived extracellular vesicles promote the migration, proliferation, and osteogenic differentiation of BMSCs.207 Researchers have successfully utilized multicellular 3D bioprinting technology to create a neural-bone construct that serves as an innervated-bone organoid. Incorporating SCs into β-calcium triphosphate tissue engineering scaffolds with BMSCs and ECs has been shown to effectively promote bone tissue regeneration through the dual effects of neurogenesis and angiogenesis.208 These findings indicate the promising research and application prospects of SCs in bone tissue engineering. Future studies should focus on understanding the regulatory effects of interactions between SCs and various cell types in bone tissue, including gene and protein expression as well as intercellular signal transduction. Exploiting the characteristics of SCs in tissue cell proliferation, secretion, adhesion, migration, and differentiation will facilitate the development of novel therapeutic strategies suitable for orthopedic clinical applications.
Endothelial cells
Bone is a highly vascularized connective tissue, and adequate vasculature is crucial for proper bone development, regeneration, and remodeling. In cases of large bone defects resulting from disease or trauma, bone grafting or bone tissue engineering approaches are necessary. However, the success of bone regeneration in both methods relies heavily on the establishment of a proper blood supply.209 Additionally, recent studies have highlighted the importance of crosstalk between ECs and neural cells in peripheral nerve regeneration.210 The blood vessels within regenerating nerves serve as pathways for Schwann cell migration, facilitating the formation of bands of Büngner that promote axonal regeneration.211 In coculture systems, ECs can promote the proliferation and migration of SCs, contributing to peripheral nerve regeneration.212 Aligned tube-like structures composed of ECs in engineered nerve constructs have been shown to enhance nerve regeneration in rat sciatic nerve models.211 VEGF, a crucial growth factor involved in angiogenesis regulation, can be secreted by ECs, and hydrogels containing VEGF have a synergistic effect with BDNF on peripheral nerve regeneration.210 Therefore, ECs also hold promise in bone tissue engineering, as they can simultaneously enhance nerve and bone regeneration. Qin et al. successfully produced a cell-laden scaffold with proangiogenic properties using 3D bioprinting technology. This was accomplished by incorporating vascular endothelial cells into a bioink based on Li-Mg-Si (LMS) bioceramics. The bioactive ions released from LMS, in conjunction with the capacity of vascular cells to secrete advantageous cytokines for neural cells, supported the differentiation of neural and osteogenic cells within the scaffolds.213
Acellular bioactive factors
Effective osteogenesis or neurogenesis necessitates the incorporation of acellular biological components, including growth factors, peptides, small molecules, DNA/RNA fragments, or exosomes, into scaffolds. These elements serve to provide supplementary stimuli that enhance neurogenesis or osteogenesis. While numerous investigations have delved into the impacts of different factors on bone or nerve tissues, there remains a dearth of acellular factors that can concurrently foster the regeneration of nerves and bones. Within this section, we delineate acellular elements that independently facilitate the regeneration of bone and nerves. Moreover, when these factors are employed in tandem, they exhibit substantial potential within the field of neuro-bone tissue engineering.
Growth factors
Biological reactions are initiated by growth factors, which are soluble signaling proteins secreted by cells. They bind to cell surface receptors and regulate intracellular signaling cascades, playing crucial roles in various cellular processes.214,215
In bone formation, several growth factors are involved, including bone morphogenetic proteins (BMPs), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF), among which BMP is widely studied.216 BMP can stimulate pluripotent cells such as BMSCs to proliferate and differentiate into chondrocytes and osteoblasts, promoting new bone formation.217 Thus, growth factors, when encapsulated within scaffolds, can be used to treat fractures effectively.181,218,219 Additionally, BMP has been shown to stimulate angiogenesis, further contributing to bone regeneration.220 Currently, BMP-2, 3, 4, 5, and 7 are the main osteogenic factors known, with BMP-2 and BMP-7 being the most extensively studied and approved by the FDA for bone regeneration.221
Nerve growth factors (NGF), brain-derived neurotrophic factors (BDNF), glial-derived neurotrophic factors (GDNF), and neurotrophic factors-3 and -4 (NT-3, NT-4) are crucial for neuronal growth and survival.222 During nerve regeneration, these neurotrophic factors play a role in enhancing stem cell differentiation, recruitment, and remyelination processes.223 The gradient of neurotrophic factors produced by nerve terminals after trauma exerts both stimulatory and inhibitory effects on nerve repair.224 Incorporating neurotrophic factors into neural conduits to promote nerve regeneration has been explored in several studies, through approaches involving either injection or modification approaches.225,226,227
Compared to conventional bone tissue engineering scaffolds, this approach, particularly with the incorporation of nerve growth factors, produces scaffolds with neuroattractive capability. The incorporation of growth factors within the scaffold offers potential for achieving the synergistic regeneration of both bone and neural components. Nevertheless, the utilization of growth factors in neuro-bone tissue engineering faces challenges such as elevated costs, unstable biological activity, and potential adverse effects.228 The high-dosage application of growth factors in attempting to enhance therapeutic effectiveness at the fracture site may precipitate inflammation, tumor formation, and ectopic bone development in unforeseen regions, especially when employed in tandem.229,230
Bioactive peptides
Incorporating bioactive peptides with active protein motifs into scaffolds offers a cost-effective alternative to the use of growth factors. Short peptide sequences derived from natural or synthetic materials stimulate cell surface receptors and regulate signaling pathways.
Peptides derived from growth factors such as osteopontin, BMP-2, and BMP-7 can be incorporated into scaffolds to promote osteogenesis in vivo.231,232,233 For instance, a modified PA66 polymer scaffold containing BMP-2-derived peptide and QK (a VEGF mimetic peptide) has successfully repaired severe femoral fractures in SD rats.234 Treatment with bone-forming peptide-1 (BFP-1), a peptide sequence from BMP-7, upregulates the expression of osteogenic genes in BMSCs, leading to greater bone formation in vivo than that elicited by the original growth factors.232 Peptides derived from the extracellular matrix that promote cell migration and proliferation are also effective tools in bone tissue engineering. Dos Santos et al. developed a growth factor-free hydrogel consisting of elastin-like polypeptides (ELPs), poly(ethylene glycol) (PEG), and a range of concentrations of the adhesion peptide IKVAV. This hydrogel promotes angiogenesis and innervation during bone repair.185
Bioactive peptides are also useful in nerve tissue engineering. Nerve conduits containing bioactive peptides that mimic the functions of BDNF and VEGF have been used to repair sciatic nerve defects in rats. These peptides enhance nerve regeneration and promote vascular penetration, leading to nerve regeneration and functional recovery.180,235 In peripheral nerves, laminin plays a crucial role in Schwann cell migration and axon extension. A self-assembling peptide nanofiber hydrogel, dual-functionalized with the laminin-derived motif IKVAV and the BDNF-mimetic peptide epitope RGI, provides a three-dimensional microenvironment for SCs and neurites, which was successfully used to bridge a 10-mm sciatic nerve defect.163 The Arg-Gly-Asp (RGD) sequence, one of the most potent ligands in natural extracellular proteins, enhances the adhesion and proliferation of SCs and promotes the axon outgrowth of dorsal root ganglions when incorporated into PCL scaffolds.236
Compared with proteins, peptides have unique advantages. Their chemical structure can be precisely controlled, they are more tolerant to temperature and pH changes, and they are more convenient to use. To date, bioactive peptides have been widely used in the field of nerve regeneration and bone regeneration, but how to use these peptides in neuro-bone tissue engineering has not been studied. This approach provides new opportunities for future research and application.
Small molecules
Small molecules, referring to natural or synthetic compounds with low molecular weights, exert therapeutic effects by modulating cellular functions.237 These molecules, characterized by their uncharged or hydrophobic properties, can penetrate the phospholipid bilayer of the cellular membrane.238
Osteoinductive molecules have been found to influence various signaling pathways within cells, including BMP signaling, the hedgehog (Hh) pathway, and Wnt/beta-catenin or cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) signaling pathways.237 For instance, the small molecule SVAK-12 has been shown to potentiate the osteogenic differentiation of myoblasts induced by BMP-2 in vitro. Moreover, the percutaneous injection of SVAK-12 has been found to accelerate rat femoral fracture callus formation.239,240 The effects of statins, cholesterol-lowering drugs, on bone formation have also been observed both in vitro and in vivo over several years.241 Recent research has demonstrated the potential of aspirin, a common drug for relieving minor aches, pains, and fevers, in promoting bone regeneration.242
Although the use of small molecules in peripheral nerve regeneration is limited, they show promise in enhancing neuronal viability, supporting axonal outgrowth, and influencing the neuronal differentiation of stem cells.5 For example, GSK-J1, LDN193189, SB431542, CHIR99021, and P7C3-A20 have documented potential to induce the neural differentiation of stem cells.243,244 Additionally, small molecules such as LM22B-10, FK506, BT13, and Lycium barbarum polysaccharides (LBP) have been shown to enhance nerve regeneration both in vivo and in vitro.245,246,247,248 On the other hand, drugs that compete with neurotransmitters that negatively affect osteogenesis can also be beneficial for bone regeneration. Propranolol, an adrenergic β-receptor blocker, competes with NE released by overactive sympathetic nerves, effectively blocking its negative effects on osteogenic differentiation. Similarly, nifedipine, a calcium channel blocker, promotes bone formation by inhibiting the release of NE.145,249,250
Small-molecule drugs have garnered significant interest as potential substitutes for growth factors due to their ease of synthesis, regulatory effects, and broad applicability. They can facilitate tissue regeneration by modulating biological pathways, although their use in nerve regeneration requires further investigation and clinical validation. Nonetheless, this offers a promising avenue for collaborative repair in nerve–bone tissue engineering.
DNA/RNA fragments
Although both growth factors and bioactive peptides can stimulate bone and nerve regeneration, the exogenous introduction of these factors often results in decreased activity, a short half-life, and the need for repeated high-dose administration. To address these challenges, the implantation of DNA/RNA fragments into scaffolds can be utilized as an alternative approach to avoid the use of high concentrations of growth factors.
Delivery of DNA fragments can be achieved through viral or nonviral vectors to target cells and modify their gene expression. However, the permanent integration of gene sequences into the host genome using viral vectors such as retroviral and lentiviral vectors is undesirable due to potential adverse effects.251 Nonviral approaches are considered safer, as they have lower immunogenicity and exert only transient effects on gene expression compared to viral delivery. Loading plasmid DNA (pDNA) into cationic scaffolds or nanoparticles appears to be a potential solution to the difficulty of transporting negatively charged pDNA across cell membranes.252 Introducing BMP-2 plasmid DNA into bone defects can provide a continuous source of growth factors to upregulate bone formation-related genes.253 VEGF plasmids and NGF plasmids loaded onto 3D-printed porous bone scaffolds can effectively replace seed cells and growth factors, inducing minimal immune response and promoting vascularized bone and nerve regeneration.254,255 The injection of a plasmid encoding fibroblast growth factor 2 (FGF2) around peripheral nerves has also shown potential in facilitating the regeneration of the sciatic nerve.256 Furthermore, the delivery of plasmids encoding miR-200c using CaCO3 nanoparticles resulted in the successful repair of craniofacial bone defects.257
In addition to DNA sequences, RNA delivery can also be employed to hinder the translation of mRNA sequences that impede bone or nerve regeneration. MicroRNAs (miRNAs) are small, naturally occurring noncoding RNAs that regulate gene expression by modulating posttranscriptional processes. Numerous miRNAs have been identified as key regulators of neurobiological processes, including neurite outgrowth, synaptogenesis, and neural plasticity. Injecting a thermoresponsive hydrogel for the codelivery of microRNA-222 and aspirin (miR222/MSN/ASP hydrogel) promoted the neuronal differentiation of BMSCs and functioned synergistically with aspirin to enhance osteogenesis.183 Loading miR-29b onto a 3D-printed scaffold with sustained release capabilities also demonstrated the promotion of bone regeneration, similar to the effects of other bioactive molecules in bone tissue engineering.258 Similarly, siRNA has been utilized to silence specific genes that inhibit osteocyte formation or facilitate osteoclast formation with high specificity.259,260,261 The use of siRNA targeting fidgetin-like 2 (FL2), a negative regulator of axon regeneration, can significantly enhance functional nerve recovery.262
Integrating DNA/RNA fragments into scaffolds to facilitate the synergistic regeneration of both bone and nerves holds significant promise and warrants further exploration.
Extracellular vesicles
Almost all types of cells secrete extracellular vesicles (EVs) that contain multiple signaling factors, such as proteins, lipids, mRNAs, miRNAs, and other noncoding RNAs, enabling communication between neighboring or distant cells during the natural healing process.263 These ingenious carriers, specifically those derived from SCs, MSCs, and macrophages, which are associated with bone and nerve regeneration, have been investigated for therapeutic use. The lipid bilayer protects the cargoes within EVs from degradation within the extracellular environment.264
In the context of bone regeneration, incorporating Schwann cell-derived exosomes into hydrogels as a multifunctional neuromodulation platform can orchestrate the bone microenvironment through immunomodulation, angiogenesis, and osteogenesis.265 Similarly, Su et al. developed exosome@aptamer (EA) constructs by coupling aptamers targeting phosphatidylserine (PS) with repair Schwann cell exosomes, which were loaded onto the surface of electrospun fibers to create a biomimetic periosteum with the ability to promote nerve and bone regeneration.266 Additionally, combining Schwann cell-derived exosomes with porous Ti6Al4V scaffolds or hydrogels has been shown to effectively improve the efficacy of scaffolds for bone defect repair in vivo.267,268 Similarly, the incorporation of MSC-derived EVs into scaffolds has been demonstrated to enhance bone repair in rodent calvarial bone defects and promote osteogenesis even at ectopic sites.189,269 Porous 3D PLA scaffolds coated with MSC-Exo have shown immunoregulatory and osteogenic effects, reducing proinflammatory marker expression and promoting osteogenic differentiation.270 Furthermore, macrophage-derived exosomes can promote the differentiation of BMSCs toward an osteoblastic fate through microRNA-21a-5p.271 Biomimetic mineralized collagen can mediate endogenous bone regeneration by recruiting MSCs and increasing the secretion of extracellular vesicles by macrophages.272
Extracellular vesicles secreted by various cells have also been shown to promote nerve regeneration. For example, SCs communicate with neighboring axons during regenerative processes via exosomes, which significantly increase axonal regeneration in vitro and enhance regeneration after sciatic nerve injury in vivo.273 BMSC-derived exosomes have been found to promote the regeneration of peripheral nerves through the miRNA-mediated regulation of regeneration-related genes in a dose-dependent manner.274,275 Exosomes from macrophages, which contain active NADPH oxidase 2 (NOX2) complexes, can be taken up by DRGs via endocytosis during nerve regeneration, playing a necessary role in neurite growth by mediating ROS signaling.276
As EVs have been employed in both bone and nerve repair, researchers are presently directing their efforts toward the identification of EVs that promote synergistic healing in bone and intrabone nerves.
Strategies in neuro-bone tissue engineering
As a potential branch of bone tissue engineering, neuro-bone tissue engineering combines neural tissue engineering and bone tissue engineering, emphasizing the importance of neural repair in bone regeneration and the crosstalk between bone and nerves during this process. Several strategies have been explored (Table 2, Fig. 7), including (i) controlling the surface morphology and structures of scaffolds; (ii) incorporating neurotrophic factors and neuropeptides into the scaffolds; (iii) using ion-doped materials to enhance nerve regeneration; (iv) adding conductive additives to facilitate neural signaling; (v) constructing coculturing cell systems to promote neural and bone cell interactions; and (vi) applying external field stimuli to stimulate neural growth and response.
Schematic representation of neuro-bone tissue engineering strategies. a Regulate cell differentiation by controlling the surface morphology and structures of scaffolds; (b) regulate cellular behavior by modifying scaffolds with neural growth factor/neuropeptides. c Incorporate bioactive ions that can influence cellular behavior into scaffolds. d Scaffolds containing electroactive nanoparticles. e Coculture systems mimic the crosstalk between different cells during bone regeneration. f External field stimulation activates endogenous neurogenic repair signals, including skeletal interoception (left) and neuropeptide secretion (right). Created with BioRender.com
Surface morphology/micro-nanostructures
In the field of tissue engineering, the use of microstructured scaffolds has attracted significant attention for promoting bone and nerve regeneration.277 Researchers have focused on mimicking complex anatomical morphologies and incorporating desired physical properties into scaffold designs.278 The stiffness, roughness, and porosity of bone materials have been found to influence the proliferation and neuronal differentiation of stem cells1 (Fig. 7a).
Notably, the neural differentiation and osteogenic differentiation of stem cells differ, and some processes are more favored on softer matrices.279,280,281 Even subtle differences in stiffness can lead to the differentiation of neural stem cells into different types of neurons, such as neurons, oligodendrocytes, and astrocytes.282,283 For instance, when the stiffness of the substrate material is below 500 Pa, neural stem cells tend to differentiate into neurons, while stiffer materials promote the differentiation of astrocytes or oligodendrocytes.284 However, it is important to note that excessively soft materials may hinder neural cell differentiation.282 Therefore, determining the optimal stiffness level for bone biomaterials is crucial to enhance the growth and activity of both bone and neural cells in engineered bone biomaterials.
Neuronal cells possess the ability to sense surface topography and exhibit diverse responses to different levels of roughness and micropatterns.285 When human neuroblastoma cells are cultured on gold substrates, the cell viability and spreading ability decrease significantly as the surface roughness increases from flat to an average roughness of approximately 100 nm.285 This decrease can be attributed to the increased hydrophilicity of rough surfaces, which reduces the adsorption of hydrophobic neuronal cell adhesion proteins.286 Moreover, neurons grown on rough substrates exhibit disrupted polarity of the actin cytoskeleton, nuclear condensation, and impaired functionality.285 Recent research has provided evidence that oriented nanofibers can replicate the topographical signals of fibronectin, enabling the precise manipulation of cell alignment and phenotypic manifestation. Scaffolds composed of silk fibroin nanofibers and designed to mimic the structure of the extracellular matrix not only promote the directional growth of neurons but also support the growth, migration, and organization of endothelial cells.287,288,289 Additionally, uniform longitudinally oriented microchannels have been found to support nerve growth,290 and ring-shaped arrays of nanopillars can attract nearby nerves to grow along the arrays.291 Li et al. pioneered the use of neodymium-doped whitlockite (Nd@WH) integrated into a double-layer poly(caprolactone) nanofiber membrane with a surface-oriented structure. The micropattern of the inner layer-oriented structure provides sufficient space for cell arrangement, bone formation, neurogenesis, and angiogenesis.292 Thus, precise control over the nanoscale roughness and arrangement of bone biomaterials is critical for regulating the growth of neuronal cells in the bone matrix.
Another crucial factor influencing the axonal growth of neuronal cells is the porous structure of biomaterials. Porous substrates provide increased surface area, making them more likely to bind with neurogenic proteins. Studies have shown that DRG neurons are more likely to develop axons when cultured on mesoporous substrates with pores approximately 300 nm in size than when cultured on flat surfaces.293 Similarly, neuroblastoma cells exhibit enhanced spreading morphology and cell density on nanoporous silicon substrates, with further improvement observed as the pore size decreases from 20 nm to 5 nm.293,294 However, there is no significant difference in the axonal growth of DRG neurons compared to that on a flat surface when the pore size increases to the micrometer range.293
Due to the heterogeneous distribution of blood vessels and nerves within bone tissue, natural bone exhibits inherent heterogeneity that is challenging to replicate. Simulating natural structures at the macroscale provides a feasible approach to meet the regenerative needs of multiple tissues. For example, allograft healing can be accelerated by incorporating matrix metalloproteinase (MMP)-degradable hydrogel-modified constructs, which mimic the natural periosteally mediated healing process by supporting endogenous cell migration and neurovascular network formation.295 Similarly, printing a tree-like bioceramic scaffold can create, through the precise programming of blade intervals and divergence angles, a spatial microenvironment that facilitates multicellular crosstalk and innervated bone regeneration.296 Haversian system-mimicking 3D scaffolds have been shown to induce osteogenic, angiogenic, and neurogenic differentiation in vitro while also accelerating the ingrowth of blood vessels and new bone formation in vivo.297 Notably, silk fibroin nanofiber scaffolds that simulate the extracellular matrix structure can induce the directional growth of neurons and facilitate the growth, migration, and arrangement of endothelial cells.287,288,289
Modification with neurotrophic factors/neuropeptides
Numerous studies have extensively investigated the crucial role of reinnervation in bone regeneration by delivering neuronal growth factors, with a particular focus on NGF and BDNF (Table 3). Moreover, the application of a coaxial nanofibrous scaffold containing NGF has been found to create a neurotrophic microenvironment that accelerates the neural differentiation of stem cells, thereby enhancing the process of osteogenesis.298 During this process, regenerated nerves induced by sustained NGF can act as mediators in the formation of ossification centers through the NGF-TrkA signaling pathway. These sensory nerves also exhibit a regulatory effect on excessive bone formation induced by BMP-2.299 Previous investigations have demonstrated that the delivery of BDNF by collagen sponges or hyaluronic acid hydrogels resulted in increased bone formation compared to the control groups.300,301,302 Therefore, the loading of various neurotrophic factors and growth factors into scaffolds for bone tissue engineering represents an effective method for treating osteonecrosis and large segmental bone defects (Fig. 7b). Nevertheless, it is important to acknowledge that these bioactive factors exhibit poor pharmacokinetic properties and are susceptible to protein degradation, thus limiting their potential as therapeutic agents.
It is widely recognized that neurogenic factors, including neurotransmitters and neuropeptides, play crucial roles in mediating the interaction between nerves and bone during the process of bone regeneration. Consequently, incorporating neurogenic factors into modified materials can mimic the positive effects of nerves on bone regeneration (Table 3). Osteogenesis-related peptides, in comparison to various macromolecular growth factors, offer several advantages, such as high efficiency, ease of synthesis, low cost, and convenient implantation in the extracellular matrix. These peptides promote cell adhesion and accelerate mineralization.303 Furthermore, neuropeptides possess low toxicity, high biological activity, and high specificity, making them highly suitable for clinical translation.304 Among neuropeptides, CGRP is widely recognized for its anabolic properties, making it a favorable candidate for introduction into bone regeneration systems to temporarily replace the function of nerves during the early stages of bone healing. However, bone regeneration is a long-term process that necessitates the continuous involvement of growth factors at each stage.2 Therefore, the design of bioactive scaffolds revolves around maintaining the controlled release of highly active peptides. Alternatively, efforts can be made to integrate transgenic cells into the scaffold to establish a stable source of neuropeptides. While the stable release of neuropeptides is crucial for promoting osteogenesis, the cell-free strategy appears to be more practical and feasible for application and development than the use of scaffolds containing transgenic cells, as the cell-free approach effectively circumvents immune rejection and ethical concerns.5 Apart from CGRP, other neuropeptides, such as VIP, SP, and Sema 3 A, have shown potential for application in bone tissue engineering. While the introduction of exogenous neurogenic factors has been shown to enhance the repair of bone defects, it is important to note that their biological effects are difficult to control due to the complex humoral environment and the widespread expression of receptors. Inaccurate and continuous delivery may lead to unexpected side effects. Therefore, future bioactive materials are expected to dynamically mimic the biological effects of nerves on bone during the bone healing process.
Ion-doped materials
The incorporation of bioactive ions into materials has shown promising results in enhancing neurogenic and osteogenic markers and activating neuromodulation in bone. The role of metal ions such as magnesium ion (Mg2+), zinc ion (Zn2+), and copper ion (Cu2+) in bone growth and remodeling has been recognized since the late 1990s.305,306 Over the past few decades, research has revealed the regulatory effects of cations on osteogenesis, osteoclastogenesis, angiogenesis, and immune responses.307,308 However, the involvement of the nervous system in the formation of new bone induced by metal ions has only recently gained attention.153 Different ions play crucial roles in various biochemical functions that are essential for different stages of bone regeneration. Their presence and influence help maintain a delicate balance between bone and neural cells (Fig. 7c). Among metal ions, magnesium has shown good bone regeneration and nerve regeneration capabilities in recent studies. As a commonly occurring element in nature, magnesium is abundant in the human body.309 Biodegradable magnesium implants exhibit excellent biocompatibility and bioactivity compared to other synthetic polymers.310 Recent studies have revealed that Mg2+ released from implants can promote the secretion of CGRP from sensory nerve endings within the bone.90,97,311,312 Additionally, Mg2+, Zn2+, and Cu2+ were found to activate skeletal interoception by modulating macrophage activity.158 Zn2+ and Cu2+ ions were also found to play crucial roles in modulating the biological activity of neurotrophic factors, which are important for nerve survival and regeneration. Zn2+ treatment in neuronal cell cultures has been associated with increased binding to BDNF and with enhanced proliferation, while the presence of Cu2+ synergistically affects nerve cell activity with NGF.222 Apart from metal ions, other inorganic ions also play a role in nerve-induced bone regeneration. For instance, silicon, an essential element for the human body, is necessary for bone homeostasis.313 Silicate glass has been used as a bioactive material due to its positive effects on osteoblasts, osteoclasts, and endothelial cells.314 However, a recent study provided new insight into silicate-based material-induced bone regeneration by triggering the release of Sema3A from DRG, which promoted rat femoral defect regeneration.315 Current bone substitute materials primarily offer osteoconductive healing properties, and many advanced tissue engineering strategies are not yet practical for everyday clinical use. The comprehensive examination of the physiological mechanisms of various ions and their impact on bone tissue regeneration indicates that incorporating certain ions into existing bone substitutes can potentially modify inflammation, the immune response, bone regeneration, and nerve regeneration. The utilization and combination of ions with existing biomaterials are influenced by various factors. The concentration of released metal ions has been shown to be critical for the process of bone formation. Therefore, it is advantageous to have these ions near the implanted biomaterial, facilitating bone regeneration directly at the implant site.
Electroactive nanoparticles
Numerous physiological functions are linked to endogenous electric fields (EFs). These functions encompass the development and organization of tissues, as well as their regeneration after injuries.316 Given the sensitivity of bone lineage cells and neural cells to electrical stimulation, the employment of electroactive materials could prove beneficial in facilitating the repair of both bone and nerve tissue317(Fig. 7d).
Over a century has passed since the first synthesis of black phosphorus (BP), which is recognized as the most stable allotrope of phosphorus.318 BP nanomaterials possess exceptional optical and mechanical properties, along with electrical conductivity, biocompatibility, and biodegradability, making them highly suitable for various biomedical applications.319 By incorporating BP into a biomimetic periosteum or hydrogel, the regeneration of nerves and bones could be stimulated, creating a favorable microenvironment for tissue repair.191,192,320 Through the application of electrical stimuli to MSCs cultured on BP@PDA hydrogels, significant improvements in cell migration and neural differentiation within 3D scaffolds have been observed.321 Furthermore, the continuous release of phosphorus ions from black phosphorus during degradation actively promotes bone mineralization and the osteogenic differentiation of mesenchymal stem cells.322,323
Polypyrrole (PPy), an organic polymer, is formed through the oxidative polymerization of pyrrole.324 Because of its excellent conductivity and antioxidation properties, PPy shows significant potential for applications in tissue engineering. The modification of scaffolds with PPy has been found to improve their mechanical properties, electrical conductivity, osteoconductivity, and drug delivery capabilities and to exert a positive effect on the growth and osteogenic differentiation of bone marrow stem cells.325,326 Furthermore, hydrogels incorporating PPy have been reported to exhibit beneficial bioactivity, electrical conductivity, and antioxidant properties, which help to create a neuroprotective and neuroinducible 3D environment for encapsulated BMSCs, thereby accelerating recovery from spinal cord injury.327,328
Polyaniline (PAni), a semiflexible rod polymer, demonstrates outstanding conductivity and serves as an organic semiconductor.329 Consequently, PLA scaffolds incorporating well-distributed PAni can offer an optimal environment for the growth and differentiation of BMSCs due to their conductive properties.325 Furthermore, coating medical titanium sheets with PAni can synergistically enhance cell proliferation and osteogenesis through electrical stimulation.330 Through the complexation of gold nanoparticles with PAni, reinforced nanocomposites can be delivered intracellularly and induce the differentiation of stem cells into neural cell lineages under electrical stimulation using an electroporator.331 Additionally, composite hydrogels containing PAni-modified carboxymethyl chitosan, when incorporated within a nerve conduit, significantly promoted sciatic nerve regeneration and Schwann cell proliferation compared to hollow chitosan conduits.332
Carbon-based materials such as graphene and carbon nanotubes are commonly used as conductive additives in bone and nerve tissue engineering due to their favorable mechanical properties, lack of cytotoxicity toward osteoblasts, and intrinsic antibacterial activity.333 The incorporation of reduced graphene oxide (rGO) into hydrogels increased the electrical conductivity of scaffolds, enhanced the osteogenic and neurogenic differentiation of loaded cells, and accelerated bone regeneration by modulating the diabetic inflammatory microenvironment.334,335 Similarly, scaffolds or nerve conduits fabricated by combining PLC and carbon nanotubes have been applied to support the ingrowth of subchondral bone and consequently the repair of 10 mm sciatic nerve defects in rats.336,337,338
Overall, scaffolds based on conductive materials offer the potential to harness the benefits of biological electric fields in bone and nerve tissue while also providing a substrate that promotes faster cell proliferation than that achieved with conventional synthetic materials. However, further research is necessary to explore the long-term capabilities of implanted conductive and electroactive bone scaffolds.
Cell coculture systems
Physiological tissues consist of multicellular systems comprising different cell types that interact with each other to facilitate viability, proliferation, and development. In recent years, tissue engineering has been moving toward cocultures, as they offer a more physiologically relevant representation of natural tissues, both physically and biologically.339 Compared to traditional monolayer cell culture, coculture systems closely mimic the in vivo environment, allowing better observation of cell‒cell interactions, where cells serve as stimulus sources for providing desired signals to other cell types340 (Fig. 7e).
Coculturing neural cells with pluripotent stem cells has shown positive effects on neurogenesis, offering an alternative strategy for reinnervation. The introduction of cocultured SCs and adipose-derived stem cells (ADSCs) as seed cells into a silk fibroin (SF)/collagen scaffold to construct a tissue-engineered nerve conduit has demonstrated improved regenerative microenvironments and accelerated nerve regeneration.341,342 Combining stem cells with ECs in coculture not only promotes DRG axon growth but also enhances neuronal orientation, potentially reducing the risk of axonal tangling and inhibiting neurofibroma formation.343 Coculturing SCs and neural stem cells (NSCs) in laminin-chitosan-poly-lactic-co-glycolic acid (laminin-chitosan-PLGA) nerve conduits has shown potential in promoting injured nerve regeneration in rats.344
In the context of bone regeneration, coculturing ECs with osteoblasts is often employed to induce capillary formation, while ECs similarly affect the phenotypic expression and proliferation of osteoblasts.345 Cocultures of BMSCs and ECs have been reported to enhance the expression of osteogenic markers and improve the proliferation of stem cells.346 Furthermore, extracellular matrices derived from coculture systems of mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVECs) have shown significant enhancements in cell proliferation compared to that achieved on scaffolds made of PCL alone while maintaining similar physical and mechanical properties.347 When preosteoblasts are cocultured with neural progenitor cells in a 3D nanocomposite hydrogel incorporating whitlockite, both the neural and osteogenic activities of the coculture system are enhanced.348
Stem cells offer remarkable versatility in tissue engineering, allowing the creation of complex tissues and organs with enhanced regenerative potential. However, ethical concerns, regulatory restrictions, immune rejection risks, and potential tumorigenicity pose significant challenges.349 In contrast, the integration of multiple cell types in tissue engineering holds promise for replicating natural tissue interactions and enhancing functionality but entails complexities in managing diverse cell populations and in obtaining regulatory approval.339 However, the increasing popularity of coculture systems in tissue engineering highlights the importance of addressing challenges such as appropriate cell selection, the optimization of cell ratios and numbers, and the refinement of culture media to significantly impact experimental outcomes.350 Furthermore, ensuring the survival of these seed cells under hypoxic conditions in vivo before complete vascularization is established remains crucial for successful tissue engineering endeavors.175
External field stimuli
The nervous system can respond to external stimulation, leading to changes in cellular behavior that can be leveraged for therapeutic purposes. Current research focuses on initiating endogenous neurogenic repair signals. One widely recognized anabolic neuropeptide, CGRP, has shown promise for enhancing bone repair when its expression is enhanced in the microenvironment. Typically, the release of CGRP is increased in response to heat, low pH, and TRPV1 agonists.351,352 However, these microenvironmental conditions can impair normal bone healing, making it impractical to translate these strategies directly. As alternatives, strategies such as electrical stimulation and low-intensity pulsed ultrasound (LIPUS) have been developed.2 These approaches aim to stimulate the nervous system and elicit beneficial effects on bone repair without the need for extreme microenvironmental conditions.
Electrical stimulation (ES) is a widely researched physical therapy modality that facilitates the repair and regeneration of damaged tissues, including bone, muscle, skin, and nerve.353 However, the parameters and strategies of electrical stimulation in neuro-bone tissue engineering may be different from those used for bone or nerve regeneration alone. In previous studies, electric or electromagnetic fields were applied directly to the fracture site to modulate local biological functions involved in the healing process.354 For bone regeneration, researchers usually use specific electrical stimulation parameters, such as a frequency of 15 hertz, an electric field intensity of 200 mV·mm−1, and a treatment time in the range of 2–8 h.353 To optimize neural tissue regeneration, the parameter settings for electrical stimulation are aimed at regulating the growth and signal transduction of nerve cells. For example, primary mouse hippocampal neurons and PC-12 cells cultivated on a scaffold with optimal electrical stimulation (100-150 mV·cm−1 for 1 h daily) exhibited augmented growth of both neurites and microfibers.355 However, electrical stimulation strategies in neuro-bone tissue engineering mainly focus on stimulating nerve cells to regulate the synthesis of specific neuropeptides, such as CGRP. It was reported that applying a maximum stimulation voltage that did not cause significant contraction of the innervated muscle (250 mV, 90 μs, 150 Hz, 61.3 μA) enhanced CGRP expression in DRG neurons.356 The implantation of electrodes (10 V, 500 μs, and 10 Hz) into the lumbar DRG was found to improve innervation of the area of femoral fracture in rats, stimulate CGRP biosynthesis and release, and ultimately accelerate fracture healing.98 These studies demonstrated that a wide range of electrical stimulation parameters can be used to stimulate CGRP+ neurons. The use of the above electrical stimulation parameters and strategies holds promise for the future development of implants with built-in electric fields that can both promote bone and nerve regeneration through electric fields and deliver electrical stimulation to the DRG to promote CGRP-mediated bone regeneration through neuromodulation. Thus, these findings provide valuable insights into the therapeutic potential of neuro-bone tissue engineering.
Low-intensity pulsed ultrasound (LIPUS) is a commonly used physical therapy method that has shown potential for promoting fracture healing357 (Fig. 7f). Unlike high-intensity ultrasound energy, LIPUS is nonthermal and nondestructive, making it a promising approach for bone tissue repair and regeneration.358 Recent studies have demonstrated that LIPUS can effectively enhance Schwann cell proliferation and axonal remyelination, thereby promoting nerve regeneration 304,403. Moreover, LIPUS has been shown to enhance CGRP-positive sensory innervation, which contributes to osteogenesis, as observed in an animal model of spinal fusion.359 However, in a rat model of tibial fracture after sciatic nerve resection, LIPUS did not promote fracture healing.360 These findings suggest that the positive effects of LIPUS on bone regeneration may be mediated by promoting CGRP secretion from sensory nerve fibers. Additionally, LIPUS has been found to increase the expression of cyclooxygenase 2 (COX-2), a key enzyme involved in the synthesis of PGE2, in osteoblasts. Elevated levels of PGE2 in the local microenvironment can activate skeletal interoception, thereby accelerating bone regeneration through neuronal pathways.361,362,363
Summary and future prospects
In this review, we delve into the influence of the nervous system on tissue regeneration from lower to higher species. We begin by providing an overview of the anatomical aspects of the nervous system within skeletal tissue and delve into the regulatory mechanisms relevant to skeletal diseases. Our focus is on addressing critical bone defect regeneration. Specifically, we emphasize the neuroendocrine and signaling functions of nerve fibers within bone tissue and their important role in fostering osteogenesis through intercellular communication. Then, we elaborated on the concept of neuro-bone tissue engineering and its basic elements, including scaffold materials, seed cells and acellular active factors, important design criteria and the latest achievements in developing neuro-bone tissue engineering materials. Finally, we combined the factors involved in neuro-bone tissue engineering to obtain six feasible strategies. In conclusion, nerve fibers enhance bone regeneration by providing neuropeptides that regulate angiogenesis, osteogenic differentiation, and immunity, and accordingly, critical bone defects can be effectively repaired by promoting the synergistic regeneration of nerve and bone or by mobilizing endogenous neurogenic repair signals.
While significant advances have been made in developing organic and inorganic materials that closely emulate the physicochemical characteristics of bone, the field of neuro-bone tissue engineering grafts is still in its nascent phase. A major obstacle involves establishing an ideal environment that facilitates the differentiation and proliferation of both nerve and bone cells within bone implant materials.
Given the highly vascularized and innervated nature of human bone, it is crucial for bone implant materials to emulate the microenvironment of bone tissue to enhance fracture healing. Recognizing the crucial role of nerves in promoting bone healing has revealed the need for deeper insights into the mechanisms underlying the interactions between nerve fibers and different cell types within skeletal tissue. Consequently, this comprehensive review aims to provide a holistic understanding of the anatomy and functional aspects of nerves in bone tissue, with the goal of advancing the field of neuro-bone tissue engineering and facilitating its clinical translation.
References
Marrella, A. et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today (Kidlington, Engl.) 21, 362–376 (2018).
Salhotra, A., Shah, H. N., Levi, B. & Longaker, M. T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 21, 696–711 (2020).
Liu, M. et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 5, 17014 (2017).
Koons, G. L., Diba, M. & Mikos, A. G. Materials design for bone-tissue engineering. Nat. Rev. Materials 5, 584–603 (2020).
Wan, Q. et al. Simultaneous regeneration of bone and nerves through materials and architectural design: are we there yet. Adv. Funct. Mater. 30, 2003542 (2020).
Qin, Q. et al. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 54, 1844–1849 (2022).
Burger, M. G. et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 149, 111–125 (2022).
Liu, S. et al. Nerves within bone and their application in tissue engineering of bone regeneration. Front. Neurol. 13, 1085560 (2022).
Gajda, M., Adriaensen, D. & Cichocki, T. Development of the innervation of long bones: expression of the growth-associated protein 43. Folia Histochem. Cytobiol. 38, 103–110 (2000).
Li, Z. et al. Fracture repair requires TrkA signaling by skeletal sensory nerves. J. Clin. Invest. 129, 5137–5150 (2019).
Tomlinson, R. E. et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 16, 2723–2735 (2016).
Xu, J. et al. NGF-p75 signaling coordinates skeletal cell migration during bone repair. Sci. Adv. 8, eabl5716 (2022).
Tao, R. et al. Hallmarks of peripheral nerve function in bone regeneration. Bone Res. 11, 6 (2023).
Leroux, A., Paiva Dos Santos, B., Leng, J., Oliveira, H. & Amédée, J. Sensory neurons from dorsal root ganglia regulate endothelial cell function in extracellular matrix remodelling. Cell Commun. Signal. 18, 162 (2020).
Zhang, Y. & Haga, N. Skeletal complications in congenital insensitivity to pain with anhidrosis: a case series of 14 patients and review of articles published in Japanese. J. Orthop. Sci. 19, 827–831 (2014).
Zhu, S. et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 129, 1076–1093 (2019).
Simon, A. & Tanaka, E. M. Limb regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2, 291–300 (2013).
Gerber, T. et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science. 362, eaaq0681 (2018).
Cao, Z. et al. Calcineurin controls proximodistal blastema polarity in zebrafish fin regeneration. Proc. Natl. Acad. Sci. USA. 118, e2009539118 (2021).
Stocum, D. L. The role of peripheral nerves in urodele limb regeneration. Eur. J. Neurosci. 34, 908–916 (2011).
Endo, T., Bryant, S. V. & Gardiner, D. M. A stepwise model system for limb regeneration. Dev. Biol. 270, 135–145 (2004).
Kumar, A. & Brockes, J. P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 35, 691–699 (2012).
Dolan, C. P. et al. Axonal regrowth is impaired during digit tip regeneration in mice. Dev. Biol. 445, 237–244 (2019).
Xu, Y. et al. Inferior alveolar nerve transection disturbs innate immune responses and bone healing after tooth extraction. Ann. N. Y. Acad. Sci. 1448, 52–64 (2019).
Cao, J. et al. Sensory nerves affect bone regeneration in rabbit mandibular distraction osteogenesis. Int J. Med Sci. 16, 831–837 (2019).
Felgueiras, H. P. Emerging antimicrobial and immunomodulatory fiber-based scaffolding systems for treating diabetic foot ulcers. Pharmaceutics. 15, 258 (2023).
Louiselle, A. E., Niemiec, S. M., Zgheib, C. & Liechty, K. W. Macrophage polarization and diabetic wound healing. Transl. Res. 236, 109–116 (2021).
Nowak, N. C., Menichella, D. M., Miller, R. & Paller, A. S. Cutaneous innervation in impaired diabetic wound healing. Transl. Res. 236, 87–108 (2021).
Yu, F. X. et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin Eye Res. 89, 101039 (2022).
Leal, E. C. et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 185, 1638–1648 (2015).
Zhang, Y. et al. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes 69, 1549–1561 (2020).
Yagi, S., Hirata, M., Miyachi, Y. & Uemoto, S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int. J. Mol. Sci. 21, 8414 (2020).
Miller, B. M., Oderberg, I. M. & Goessling, W. Hepatic nervous system in development, regeneration, and disease. Hepatology 74, 3513–3522 (2021).
Tanaka, K., Ohkawa, S., Nishino, T., Niijima, A. & Inoue, S. Role of the hepatic branch of the vagus nerve in liver regeneration in rats. Am. J. Physiol. 253, G439–G444 (1987).
Izumi, T. et al. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat. Commun. 9, 5300 (2018).
Mizutani, T. et al. Calcitonin gene-related peptide regulates the early phase of liver regeneration. J. Surg. Res. 183, 138–145 (2013).
Laschinger, M. et al. The CGRP receptor component RAMP1 links sensory innervation with YAP activity in the regenerating liver. FASEB J. 34, 8125–8138 (2020).
Kim, J. H. et al. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 11, 1025 (2020).
Lepore, E., Casola, I., Dobrowolny, G. & Musarò, A. Neuromuscular junction as an entity of nerve-muscle communication. Cells. 8(2019).
Cisterna, B. A. et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat. Commun. 11, 1073 (2020).
Ciciliot, S. & Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Clin. Implic. Curr. Pharm. Des. 16, 906–914 (2010).
Liu, L., Dana, R. & Yin, J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB J. 34, 6229–6243 (2020).
Jacobsen, N. L., Morton, A. B. & Segal, S. S. Angiogenesis precedes myogenesis during regeneration following biopsy injury of skeletal muscle. Skelet. Muscle 13, 3 (2023).
Mastorakos, P. & McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 4, eaav0492 (2019).
Bajayo, A. et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl. Acad. Sci. USA. 109, 15455–15460 (2012).
Varadarajan, S. G., Hunyara, J. L., Hamilton, N. R., Kolodkin, A. L. & Huberman, A. D. Central nervous system regeneration. Cell 185, 77–94 (2022).
Xia, W. et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 12, 6043 (2021).
Shehab, D., Elgazzar, A. H. & Collier, B. D. Heterotopicossification ossification. J. Nucl. Med. 43, 346–353 (2002).
Xu, Y. et al. Heterotopic ossification: Clinical features, basic researches, and mechanical stimulations. Front. Cell Dev. Biol. 10, 770931 (2022).
Shams, R. et al. The pathophysiology of osteoporosis after spinal cord injury. Int. J. Mol. Sci. 22, 3057 (2021).
Zeng, L. et al. Guidelines for management of pediatric acute hyperextension spinal cord injury. Chin. J. Traumatol. 26, 2–7 (2023).
Zhang, L. et al. Bidirectional control of parathyroid hormone and bone mass by subfornical organ. Neuron 111, 1914–1932.e6 (2023).
Huang, S. et al. Neural regulation of bone remodeling: Identifying novel neural molecules and pathways between brain and bone. J. Cell. Physiol. 234, 5466–5477 (2019).
Zhang, Z. et al. Neuro-bone tissue engineering: Multiple potential translational strategies between nerve and bone. Acta Biomater. 153, 1–12 (2022).
Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 473, 231–236 (2008).
Dimitri, P. & Rosen, C. The central nervous system and bone metabolism: an evolving story. Calcif. Tissue Int. 100, 476–485 (2017).
Maryanovich, M., Takeishi, S. & Frenette, P. S. Neural regulation of bone and bone marrow. Cold Spring Harb Perspect Med. 8, a031344 (2018).
van Galen, K. A., Ter Horst, K. W. & Serlie, M. J. Serotonin, food intake, and obesity. Obes. Rev. 22, e13210 (2021).
Chabbi-Achengli, Y. et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc. Natl. Acad. Sci. USA. 109, 2567–2572 (2012).
Nam, S. S. et al. Serotonin inhibits osteoblast differentiation and bone regeneration in rats. J. Periodontol. 87, 461–469 (2016).
Yun, H. M., Park, K. R., Hong, J. T. & Kim, E. C. Peripheral serotonin-mediated system suppresses bone development and regeneration via serotonin 6 G-protein-coupled receptor. Sci. Rep. 6, 30985 (2016).
Yadav, V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008).
Kode, A. et al. FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin. J. Clin. Invest. 122, 3490–3503 (2012).
Yadav, V. K. et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 (2009).
Kumar, M., Wadhwa, R., Kothari, P., Trivedi, R. & Vohora, D. Differential effects of serotonin reuptake inhibitors fluoxetine and escitalopram on bone markers and microarchitecture in Wistar rats. Eur. J. Pharmacol. 825, 57–62 (2018).
Durham, E., Zhang, Y., LaRue, A., Bradshaw, A. & Cray, J. Selective serotonin reuptake inhibitors (SSRI) affect murine bone lineage cells. Life Sci. 255, 117827 (2020).
Ferretti, G. et al. An increase in Semaphorin 3A biases the axonal direction and induces an aberrant dendritic arborization in an in vitro model of human neural progenitor differentiation. Cell Biosci. 12, 182 (2022).
Hayashi, M. et al. Osteoprotection by semaphorin 3A. Nature 485, 69–74 (2012).
Kim, B. J. & Koh, J. M. Coupling factors involved in preserving bone balance. Cell. Mol. Life Sci. 76, 1243–1253 (2019).
Fukuda, T. et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 497, 490–493 (2013).
Li, Z. et al. The role of semaphorin 3A in bone remodeling. Front. Cell Neurosci. 11, 40 (2017).
Sun, S. et al. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone 131, 115109 (2020).
Pezet, S. & McMahon, S. B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538 (2006).
Su, Y. W. et al. Roles of neurotrophins in skeletal tissue formation and healing. J. Cell. Physiol. 233, 2133–2145 (2018).
Ida-Yonemochi, H., Yamada, Y., Yoshikawa, H. & Seo, K. Locally produced BDNF promotes sclerotic change in alveolar bone after nerve injury. PLoS One 12, e0169201 (2017).
Liu, Q., Lei, L., Yu, T., Jiang, T. & Kang, Y. Effect of brain-derived neurotrophic factor on the neurogenesis and osteogenesis in bone engineering. Tissue Eng. Part A 24, 1283–1292 (2018).
Zhang, Z., Hu, P., Wang, Z., Qiu, X. & Chen, Y. BDNF promoted osteoblast migration and fracture healing by up-regulating integrin β1 via TrkB-mediated ERK1/2 and AKT. Signal. J. Cell. Mol. Med. 24, 10792–10802 (2020).
Ai, L. S. et al. Inhibition of BDNF in multiple myeloma blocks osteoclastogenesis via down-regulated stroma-derived RANKL expression both in vitro and in vivo. PLoS One 7, e46287 (2012).
Wan, Q. Q. et al. Crosstalk between Bone and Nerves within Bone. Adv. Sci. (Weinh). 8, 2003390 (2021).
Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 98, 1083–1112 (2018).
Kang, H. W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319 (2016).
Li, J., Kreicbergs, A., Bergström, J., Stark, A. & Ahmed, M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J. Orthop. Res. 25, 1204–1212 (2007).
Li, J., Ahmad, T., Spetea, M., Ahmed, M. & Kreicbergs, A. Bone reinnervation after fracture: a study in the rat. J. Bone Miner. Res. 16, 1505–1510 (2001).
Pagani, F. et al. Sympathectomy alters bone architecture in adult growing rats. J. Cell. Biochem. 104, 2155–2164 (2008).
Elefteriou, F. et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005).
Burt-Pichat, B. et al. Dramatic decrease of innervation density in bone after ovariectomy. Endocrinology 146, 503–510 (2005).
Mano, T., Nishimura, N. & Iwase, S. Sympathetic neural influence on bone metabolism in microgravity (Review). Acta Physiol. Hung. 97, 354–361 (2010).
Vignaux, G., Ndong, J. D., Perrien, D. S. & Elefteriou, F. Inner ear vestibular signals regulate bone remodeling via the sympathetic nervous system. J. Bone Miner. Res. 30, 1103–1111 (2015).
Matteoli, M. et al. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc. Natl. Acad. Sci. USA 85, 7366–7370 (1988).
Zhang, Y. et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 22, 1160–1169 (2016).
Zhou, R., Yuan, Z., Liu, J. & Liu, J. Calcitonin gene-related peptide promotes the expression of osteoblastic genes and activates the WNT signal transduction pathway in bone marrow stromal stem cells. Mol. Med. Rep. 13, 4689–4696 (2016).
Li, H. et al. Corrigendum: CGRP regulates the age-related switch between osteoblast and adipocyte differentiation. Front. Cell Dev. Biol. 9, 715740 (2021).
Assefa, F. The role of sensory and sympathetic nerves in craniofacial bone regeneration. Neuropeptides 99, 102328 (2023).
Wang, L. et al. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone 46, 1369–1379 (2010).
He, H. et al. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation. Mol. Med. Rep. 13, 3977–3984 (2016).
Gaete, P. S., Lillo, M. A., Puebla, M., Poblete, I. & Figueroa, X. F. CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells. Sci. Rep. 9, 7932 (2019).
Li, Y. et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials 275, 120984 (2021).
Mi, J. et al. Implantable electrical stimulation at dorsal root ganglions accelerates osteoporotic fracture healing via calcitonin gene-related peptide. Adv. Sci. (Weinh.) 9, e2103005 (2022).
Niedermair, T., Straub, R. H., Brochhausen, C. & Gr„ssel, S. Impact of the sensory and sympathetic nervous system on fracture healing in ovariectomized mice. Int. J. Mol. Sci. 21, 405 (2020).
Yuan, Y. et al. Deficiency of calcitonin gene-related peptide affects macrophage polarization in osseointegration. Front. Physiol. 11, 733 (2020).
Duan, J. X. et al. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol. Immunol. 91, 105–113 (2017).
Yuan, K. et al. Sensory nerves promote corneal inflammation resolution via CGRP mediated transformation of macrophages to the M2 phenotype through the PI3K/AKT signaling pathway. Int. Immunopharmacol. 102, 108426 (2022).
Harrison, S. & Geppetti, P. Substance p. Int. J. Biochem. Cell Biol. 33, 555-576 (2001).
Liu, D., Jiang, L. S., Dai, L. Y. & Substance, P. and its receptors in bone metabolism. Neuropeptides 41, 271–283 (2007).
Mei, G. et al. Substance P activates the Wnt signal transduction pathway and enhances the differentiation of mouse preosteoblastic MC3T3-E1 cells. Int. J. Mol. Sci. 15, 6224–6240 (2014).
Geng, W. et al. Substance P enhances BMSC osteogenic differentiation via autophagic activation. Mol. Med. Rep. 20, 664–670 (2019).
Adamus, M. A. & Dabrowski, Z. J. Effect of the neuropeptide substance P on the rat bone marrow-derived osteogenic cells in vitro. J. Cell. Biochem. 81, 499–506 (2001).
Zhang, Y. et al. Systemic injection of substance P promotes murine calvarial repair through mobilizing endogenous mesenchymal stem cells. Sci. Rep. 8, 12996 (2018).
Liu, H. J. et al. Substance P promotes the proliferation, but inhibits differentiation and mineralization of osteoblasts from rats with spinal cord injury via RANKL/OPG system. PLoS One 11, e0165063 (2016).
Guo, X. et al. Clinical guidelines for neurorestorative therapies in spinal cord injury (2021 China version). J. Neurorestoratol. 9, 31–49 (2021).
Mori, T. et al. Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone resorption. Biochem. Biophys. Res. Commun. 262, 418–422 (1999).
Wang, L. et al. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone 45, 309–320 (2009).
Offley, S. C. et al. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J. Bone Miner. Res. 20, 257–267 (2005).
Um, J., Yu, J., Dubon, M. J. & Park, K. S. Substance P and thiorphan synergically enhance angiogenesis in wound healing. Tissue Eng. Regen. Med. 13, 149–154 (2016).
Um, J., Yu, J. & Park, K. S. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol. Med Rep. 15, 3035–3040 (2017).
Hong, J. Y. et al. Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats. J. Tissue Eng. 13, 20417314221086491 (2022).
Jiang, M. H. et al. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport 23, 786–792 (2012).
Liu, S. et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron 108, 436–450.e7 (2020).
Vähätalo, L. H., Ruohonen, S. T., Ailanen, L. & Savontaus, E. Neuropeptide Y in noradrenergic neurons induces obesity in transgenic mouse models. Neuropeptides 55, 31–37 (2016).
Xie, W. et al. Neuropeptide Y1 receptor antagonist promotes osteoporosis and microdamage repair and enhances osteogenic differentiation of bone marrow stem cells via cAMP/PKA/CREB pathway. Aging (Albany NY) 12, 8120–8136 (2020).
Lee, N. J. et al. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J. Bone Miner. Res. 25, 1736–1747 (2010).
Zhang, Y. et al. Neuronal induction of bone-fat imbalance through osteocyte neuropeptide Y. Adv. Sci. (Weinh.) 8, e2100808 (2021).
Long, H., Ahmed, M., Ackermann, P., Stark, A. & Li, J. Neuropeptide Y innervation during fracture healing and remodeling. A study of angulated tibial fractures in the rat. Acta Orthop. 81, 639–646 (2010).
Wu, J. et al. Neuropeptide Y enhances proliferation and prevents apoptosis in rat bone marrow stromal cells in association with activation of the Wnt/β-catenin pathway in vitro. Stem Cell Res. 21, 74–84 (2017).
Baldock, P. A. et al. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J. Bone Miner. Res. 29, 2238–2249 (2014).
Gu, X. C., Zhang, X. B., Hu, B., Zi, Y. & Li, M. Neuropeptide Y accelerates post-fracture bone healing by promoting osteogenesis of mesenchymal stem cells. Neuropeptides 60, 61–66 (2016).
Sousa, D. M. et al. Neuropeptide Y modulates fracture healing through Y1 receptor signaling. J. Orthop. Res. 31, 1570–1578 (2013).
Liu, S. et al. Neuropeptide Y stimulates osteoblastic differentiation and VEGF expression of bone marrow mesenchymal stem cells related to canonical Wnt signaling activating in vitro. Neuropeptides 56, 105–113 (2016).
Shi, L. et al. Function study of vasoactive intestinal peptide on chick embryonic bone development. Neuropeptides 83, 102077 (2020).
Moody, T. W., Nuche-Berenguer, B. & Jensen, R. T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 23, 38–47 (2016).
Shi, L. et al. Vasoactive intestinal peptide stimulates bone marrow-mesenchymal stem cells osteogenesis differentiation by activating Wnt/β-catenin signaling pathway and promotes rat skull defect repair. Stem Cells Dev. 29, 655–666 (2020).
Liu, X. et al. Postmenopausal osteoporosis is associated with the regulation of SP, CGRP, VIP, and NPY. Biomed. Pharmacother. 104, 742–750 (2018).
Shi, L. et al. Vasoactive intestinal peptide promotes fracture healing in sympathectomized mice. Calcif. Tissue Int. 109, 55–65 (2021).
Eger, M. et al. Therapeutic potential of vasoactive intestinal peptide and its derivative stearyl-norleucine-VIP in inflammation-induced osteolysis. Front. Pharm. 12, 638128 (2021).
Valdehita, A., Carmena, M. J., Collado, B., Prieto, J. C. & Bajo, A. M. Vasoactive intestinal peptide (VIP) increases vascular endothelial growth factor (VEGF) expression and secretion in human breast cancer cells. Regul. Pept. 144, 101–108 (2007).
Kanemitsu, M. et al. Role of vasoactive intestinal peptide in the progression of osteoarthritis through bone sclerosis and angiogenesis in subchondral bone. J. Orthop. Sci. 25, 897–906 (2020).
Wang, Y. et al. In-situ-generated vasoactive intestinal peptide loaded microspheres in mussel-inspired polycaprolactone nanosheets creating spatiotemporal releasing microenvironment to promote wound healing and angiogenesis. ACS Appl. Mater. Interfaces 8, 7411–7421 (2016).
Ivanov, E., Akhmetshina, M., Erdiakov, A. & Gavrilova, S. Sympathetic system in wound healing: Multistage control in normal and diabetic skin. Int. J. Mol. Sci. 24, 2045 (2023).
Khosla, S. et al. Sympathetic β1-adrenergic signaling contributes to regulation of human bone metabolism. J. Clin. Invest. 128, 4832–4842 (2018).
Karsenty, G. & Khosla, S. The crosstalk between bone remodeling and energy metabolism: A translational perspective. Cell Metab. 34, 805–817 (2022).
Hedderich, J. et al. Norepinephrine inhibits the proliferation of human bone marrow-derived mesenchymal stem cells via β2-adrenoceptor-mediated ERK1/2 and PKA phosphorylation. Int. J. Mol. Sci. 21, 3924 (2020).
Ma, Y. et al. 2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152, 1412–1422 (2011).
Yao, Q. et al. Beta-adrenergic signaling affect osteoclastogenesis via osteocytic MLO-Y4 cells’ RANKL production. Biochem. Biophys. Res. Commun. 488, 634–640 (2017).
Chen, H. et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 10, 181 (2019).
Wu, H. et al. Blockade of adrenergic β-receptor activation through local delivery of propranolol from a 3D collagen/polyvinyl alcohol/hydroxyapatite scaffold promotes bone repair in vivo. Cell Prolif. 53, e12725 (2020).
Haffner-Luntzer, M. et al. Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via β-AR signaling. Proc. Natl. Acad. Sci. USA 116, 8615–8622 (2019).
Sato, T. et al. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS Lett. 584, 817–824 (2010).
Huang, Q., Liao, C., Ge, F., Ao, J. & Liu, T. Acetylcholine bidirectionally regulates learning and memory. J. Neurorestoratol. 10, 100002 (2022).
Mandl, P. et al. Nicotinic acetylcholine receptors modulate osteoclastogenesis. Arthritis Res. Ther. 18, 63 (2016).
Negishi-Koga, T. & Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231, 241–256 (2009).
Ternes, S. et al. Impact of acetylcholine and nicotine on human osteoclastogenesis in vitro. Int. Immunopharmacol. 29, 215–221 (2015).
Spieker, J., Ackermann, A., Salfelder, A., Vogel-Höpker, A. & Layer, P. G. Acetylcholinesterase regulates skeletal in ovo development of chicken limbs by ACh-dependent and -independent mechanisms. PLoS One 11, e0161675 (2016).
Lv, X., Gao, F. & Cao, X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 34, 1914–1931 (2022).
Zhang, Y. Y. et al. Microsomal prostaglandin E2 synthase-1 and its inhibitors: Molecular mechanisms and therapeutic significance. Pharmacol. Res. 175, 105977 (2022).
Lisowska, B., Kosson, D. & Domaracka, K. Positives and negatives of nonsteroidal anti-inflammatory drugs in bone healing: the effects of these drugs on bone repair. Drug Des. Devel. Ther. 12, 1809–1814 (2018).
Hu, B. et al. Sensory nerves regulate mesenchymal stromal cell lineage commitment by tuning sympathetic tones. J. Clin. Invest. 130, 3483–3498 (2020).
Xue, P. et al. PGE2/EP4 skeleton interoception activity reduces vertebral endplate porosity and spinal pain with low-dose celecoxib. Bone Res. 9, 36 (2021).
Qiao, W. et al. Divalent metal cations stimulate skeleton interoception for new bone formation in mouse injury models. Nat. Commun. 13, 535 (2022).
Rousseaud, A., Moriceau, S., Ramos-Brossier, M. & Oury, F. Bone-brain crosstalk and potential associated diseases. Horm. Mol. Biol. Clin. Investig. 28, 69–83 (2016).
Pinho-Ribeiro, F. A., Verri, W. A. Jr & Chiu, I. M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 38, 5–19 (2017).
Baral, P., Udit, S. & Chiu, I. M. Pain and immunity: implications for host defence. Nat. Rev. Immunol. 19, 433–447 (2019).
Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 21, 8652 (2020).
Yang, S. et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 10, 8227–8249 (2020).
Chartier, S. R. et al. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 155, 2323–2336 (2014).
Asaumi, K., Nakanishi, T., Asahara, H., Inoue, H. & Takigawa, M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 26, 625–633 (2000).
Ding, W. G. et al. Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 159, 28–34 (2010).
Kilian, O. et al. BDNF and its TrkB receptor in human fracture healing. Ann. Anat. 196, 286–295 (2014).
Lin, Y. et al. Decreased expression of semaphorin3A/neuropilin-1 signaling axis in apical periodontitis. Biomed. Res. Int. 2017, 8724503 (2017).
Tang, P. et al. NPY and CGRP inhibitor influence on ERK pathway and macrophage aggregation during fracture healing. Cell. Physiol. Biochem. 41, 1457–1467 (2017).
Appelt, J. et al. The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine 59, 102970 (2020).
Lim, J. E., Chung, E. & Son, Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 7, 9417 (2017).
Schmidt, A. H. Autologous bone graft: is it still the gold standard. Injury 52, S18–18S22 (2021).
Hofmann, A. et al. Autologous Iliac bone graft compared with biphasic hydroxyapatite and calcium sulfate cement for the treatment of bone defects in tibial plateau fractures: A prospective, randomized, open-label, multicenter study. J. Bone Jt. Surg. Am. 102, 179–193 (2020).
Tournier, P. et al. An extrudable partially demineralized allogeneic bone paste exhibits a similar bone healing capacity as the “Gold Standard” bone graft. Front. Bioeng. Biotechnol. 9, 658853 (2021).
Steijvers, E., Ghei, A. & Xia, Z. Manufacturing artificial bone allografts: a perspective. Biomater. Transl. 3, 65–80 (2022).
Bharadwaz, A. & Jayasuriya, A. C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 110, 110698 (2020).
Borkowski, L. et al. Fluorapatite ceramics for bone tissue regeneration: Synthesis, characterization and assessment of biomedical potential. Mater. Sci. Eng. C. Mater. Biol. Appl. 116, 111211 (2020).
Guo, L. et al. The role of natural polymers in bone tissue engineering. J. Control Release 338, 571–582 (2021).
Kang, Y. et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 18, 26–41 (2022).
Rao, F. et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 10, 1590–1603 (2020).
Fitzpatrick, V. et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 276, 120995 (2021).
Toosi, S. et al. Bioactive glass-collagen/poly (glycolic acid) scaffold nanoparticles exhibit improved biological properties and enhance osteogenic lineage differentiation of mesenchymal stem cells. Front. Bioeng. Biotechnol. 10, 963996 (2022).
Lei, L. et al. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 7, 2722–2735 (2019).
Li, Y. et al. Artificial PGA/collagen-based bilayer conduit in short gap interposition setting provides comparable regenerative potential to direct suture. Plast. Reconstr. Surg. Glob. Open 11, e4875 (2023).
Dos Santos, B. P. et al. Development of a cell-free and growth factor-free hydrogel capable of inducing angiogenesis and innervation after subcutaneous implantation. Acta Biomater. 99, 154–167 (2019).
Ardhani, R., Ana, I. D. & Tabata, Y. Gelatin hydrogel membrane containing carbonate hydroxyapatite for nerve regeneration scaffold. J. Biomed. Mater. Res. A 108, 2491–2503 (2020).
Yan, X. et al. PDLLA/β-TCP/HA/CHS/NGF sustained-release conduits for peripheral nerve regeneration. J. Wuhan. Univ. Technol. Mater. Sci. Ed. 36, 600–606 (2021).
Wu, H. et al. Dynamic degradation patterns of porous polycaprolactone/β-tricalcium phosphate composites orchestrate macrophage responses and immunoregulatory bone regeneration. Bioact. Mater. 21, 595–611 (2023).
Qi, X. et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 12, 836–849 (2016).
Liu, T., Li, B., Chen, G., Ye, X. & Zhang, Y. Nano tantalum-coated 3D printed porous polylactic acid/beta-tricalcium phosphate scaffolds with enhanced biological properties for guided bone regeneration. Int. J. Biol. Macromol. 221, 371–380 (2022).
Xu, Y. et al. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 16, 271–284 (2022).
Jing, X. et al. Photosensitive and conductive hydrogel induced innerved bone regeneration for infected bone defect repair. Adv. Health. Mater. 12, e2201349 (2023).
Ye, B., Wu, B., Su, Y., Sun, T. & Guo, X. Recent advances in the application of natural and synthetic polymer-based scaffolds in musculoskeletal regeneration. Polymers. 14(2022).
Ma, Q. L. et al. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 35, 9853–9867 (2014).
Li, J. et al. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv. Sci. (Weinh.) 5, 1700678 (2018).
Kohno, Y. et al. Treating titanium particle-induced inflammation with genetically modified NF-κB sensing IL-4 secreting or preconditioned mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 5, 3032–3038 (2019).
Arthur, A. & Gronthos, S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int. J. Mol. Sci. 21(2020).
Cai, X. X., Luo, E. & Yuan, Q. Interaction between Schwann cells and osteoblasts in vitro. Int. J. Oral. Sci. 2, 74–81 (2010).
Zhang, H. et al. Calcium silicate nanowires-containing multicellular bioinks for 3D bioprinting of neural-bone constructs. Nano Today 46, 101584 (2022).
Wu, Y. et al. Pre-implanted sensory nerve could enhance the neurotization in tissue-engineered bone graft. Tissue Eng. Part A 21, 2241–2249 (2015).
Wang, S. et al. BMSC-derived extracellular matrix better optimizes the microenvironment to support nerve regeneration. Biomaterials 280, 121251 (2022).
Raoofi, A. et al. Bone marrow mesenchymal stem cell condition medium loaded on PCL nanofibrous scaffold promoted nerve regeneration after sciatic nerve transection in male rats. Neurotox. Res. 39, 1470–1486 (2021).
Jessen, K. R. & Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. (Lond.). 594, 3521–3531 (2016).
Schlosshauer, B., Müller, E., Schröder, B., Planck, H. & Müller, H. W. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 963, 321–326 (2003).
Keilhoff, G., Goihl, A., Langnäse, K., Fansa, H. & Wolf, G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur. J. Cell Biol. 85, 11–24 (2006).
Jones, R. E. et al. Skeletal stem cell-schwann cell circuitry in mandibular repair. Cell Rep. 28, 2757–2766.e5 (2019).
Wang, D. et al. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 140, 610–624 (2022).
Zhang, X. et al. Schwann cells promote prevascularization and osteogenesis of tissue-engineered bone via bone marrow mesenchymal stem cell-derived endothelial cells. Stem Cell Res. Ther. 12, 382 (2021).
Filipowska, J., Tomaszewski, K. A., Niedźwiedzki, Ł., Walocha, J. A. & Niedźwiedzki, T.The role of vasculature in bone development, regeneration and proper systemic functioning.Angiogenesis 20,291–302 (2017).
Lu, J. et al. Synergistic effects of dual-presenting VEGF- and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 11, 19943–19958 (2019).
Muangsanit, P., Roberton, V., Costa, E. & Phillips, J. B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 126, 224–237 (2021).
Meng, D. H. et al. Endothelial cells promote the proliferation and migration of Schwann cells. Ann. Transl. Med. 10, 78 (2022).
Qin, C. et al. Cell-laden scaffolds for vascular-innervated bone regeneration. Adv Healthc. Mater. 12, e2201923 (2023).
Oliveira, É. R. et al. Advances in growth factor delivery for bone tissue engineering. Int. J. Mol. Sci. 22(2021).
Legrand, J. & Martino, M. M. Growth factor and cytokine delivery systems for wound healing. Cold Spring Harb. Perspect Biol. 14(2022).
Seims, K. B., Hunt, N. K. & Chow, L. W. Strategies to control or mimic growth factor activity for bone, cartilage, and osteochondral tissue engineering. Bioconjug. Chem. 32, 861–878 (2021).
Wu, M., Chen, G. & Li, Y. P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 4, 16009 (2016).
Liu, X. et al. Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials 276, 121037 (2021).
Chai, S. et al. Injectable photo-crosslinked bioactive BMSCs-BMP2-GelMA scaffolds for bone defect repair. Front. Bioeng. Biotechnol. 10, 875363 (2022).
Pulkkinen, H. H. et al. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis 24, 129–144 (2021).
Gillman, C. E. & Jayasuriya, A. C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 130, 112466 (2021).
Nicoletti, V. G., Pajer, K., Calcagno, D., Pajenda, G. & Nógrádi, A. The role of metals in the neuroregenerative action of BDNF, GDNF, NGF and other neurotrophic factors. Biomolecules. 12(2022).
Lien, B. V. et al. Enhancing peripheral nerve regeneration with neurotrophic factors and bioengineered scaffolds: A basic science and clinical perspective. J. Peripher. Nerv. Syst. 25, 320–334 (2020).
Idrisova, K. F. et al. Application of neurotrophic and proangiogenic factors as therapy after peripheral nervous system injury. Neural Regen. Res. 17, 1240–1247 (2022).
Zhou, G. et al. Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl. Mater. Interfaces 12, 16168–16177 (2020).
Carvalho, C. R. et al. Engineering silk fibroin-based nerve conduit with neurotrophic factors for proximal protection after peripheral nerve injury. Adv. Health. Mater. 10, e2000753 (2021).
Escobar, A., Reis, R. L. & Oliveira, J. M. Nanoparticles for neurotrophic factor delivery in nerve guidance conduits for peripheral nerve repair. Nanomed. (Lond.) 17, 477–494 (2022).
Safari, B., Davaran, S. & Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 175, 544–557 (2021).
James, A. W. et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 22, 284–297 (2016).
Hashimoto, K. et al. In vivo dynamic analysis of BMP-2-induced ectopic bone formation. Sci. Rep. 10, 4751 (2020).
Gabarin, N. et al. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10-14)] and attenuation of activation by cAMP. J. Cell. Biochem. 81, 594–603 (2001).
Kim, H. K. et al. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials 33, 7057–7063 (2012).
Sun, T. et al. Loading of BMP-2-related peptide onto three-dimensional nano-hydroxyapatite scaffolds accelerates mineralization in critical-sized cranial bone defects. J. Tissue Eng. Regen. Med. 12, 864–877 (2018).
Li, A. et al. Nanohydroxyapatite/polyamide 66 crosslinked with QK and BMP-2-derived peptide prevented femur nonunion in rats. J. Mater. Chem. B 9, 2249–2265 (2021).
Li, C. et al. Polydopamine-modified chitin conduits with sustained release of bioactive peptides enhance peripheral nerve regeneration in rats. Neural Regen. Res. 17, 2544–2550 (2022).
Zhu, L. et al. Noncovalent bonding of RGD and YIGSR to an electrospun Poly(ε-Caprolactone) conduit through peptide self-assembly to synergistically promote sciatic nerve regeneration in rats. Adv. Healthc. Mater. 6(2017). https://doi.org/10.1002/adhm.201600860
Balmayor, E. R. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 94, 13–27 (2015).
Lo, K. W. Effects on bone regeneration of single-dose treatment with osteogenic small molecules. Drug Discov. Today 27, 1538–1544 (2022).
Kato, S., Sangadala, S., Tomita, K., Titus, L. & Boden, S. D. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Mol. Cell. Biochem. 349, 97–106 (2011).
Wong, E. et al. A novel low-molecular-weight compound enhances ectopic bone formation and fracture repair. J. Bone Jt. Surg. Am. 95, 454–461 (2013).
Chamani, S. et al. The role of statins in the differentiation and function of bone cells. Eur. J. Clin. Invest. 51, e13534 (2021).
Zhang, Y. et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 11, 130–139 (2022).
Raeisossadati, R. et al. Small molecule GSK-J1 affects differentiation of specific neuronal subtypes in developing rat retina. Mol. Neurobiol. 56, 1972–1983 (2019).
Yang, Y. et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 269, 120479 (2021).
Labroo, P. et al. Controlled delivery of FK506 to improve nerve regeneration. Shock 46, 154–159 (2016).
Sidorova, Y. A. et al. A novel small molecule GDNF receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front. Pharm. 8, 365 (2017).
Au, N. et al. Clinically relevant small-molecule promotes nerve repair and visual function recovery. NPJ Regen. Med. 7, 50 (2022).
Cui, Z. et al. LM22B-10 promotes corneal nerve regeneration through in vitro 3D co-culture model and in vivo corneal injury model. Acta Biomater. 146, 159–176 (2022).
Li, S. et al. Inhibition of sympathetic activation by delivering calcium channel blockers from a 3D printed scaffold to promote bone defect repair. Adv. Health. Mater. 11, e2200785 (2022).
Guo, S. & He, C. Bioprinted scaffold remodels the neuromodulatory microenvironment for enhancing bone regeneration. Adv. Funct. Mater. 2304172 (2023).
Shirley, J. L., de Jong, Y. P., Terhorst, C. & Herzog, R. W. Immune responses to viral gene therapy vectors. Mol. Ther. 28, 709–722 (2020).
Cai, Y. et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-β1. Bioact. Mater. 19, 444–457 (2023).
Li, J. et al. BMP-2 plasmid DNA-loaded chitosan films - A new strategy for bone engineering. J. Craniomaxillofac Surg. 45, 2084–2091 (2017).
Fang, Z. et al. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes. J. Nanobiotechnol. 18, 46 (2020).
Zha, Y. et al. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 11, 397–409 (2021).
Masgutov, R. et al. Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve. Neural Regen. Res 16, 1882–1889 (2021).
Remy, M. T. et al. Plasmid encoding miRNA-200c delivered by CaCO3-based nanoparticles enhances rat alveolar bone formation. Nanomed. (Lond.) 17, 1339–1354 (2022).
Pan, T. et al. MicroRNA-activated hydrogel scaffold generated by 3D printing accelerates bone regeneration. Bioact. Mater. 10, 1–14 (2022).
Manaka, T. et al. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials 32, 9642–9648 (2011).
Zhang, Y., Wei, L., Miron, R. J., Shi, B. & Bian, Z. Bone scaffolds loaded with siRNA-Semaphorin4d for the treatment of osteoporosis related bone defects. Sci. Rep. 6, 26925 (2016).
Wang, Y., Zhang, S. & Benoit, D. Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control Release 287, 58–66 (2018).
Baker, L. et al. Fidgetin-like 2 negatively regulates axonal growth and can be targeted to promote functional nerve regeneration. JCI Insight. 6(2021).
Mondal, J. et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J. Control Release 353, 1127–1149 (2023).
Kar, R. et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater. Sci. Eng. 9, 577–594 (2023).
Hao, Z. et al. A multifunctional neuromodulation platform utilizing Schwann cell-derived exosomes orchestrates bone microenvironment via immunomodulation, angiogenesis and osteogenesis. Bioact. Mater. 23, 206–222 (2023).
Su, Y. et al. Aptamer engineering exosomes loaded on biomimetic periosteum to promote angiogenesis and bone regeneration by targeting injured nerves via JNK3 MAPK pathway. Mater. Today Bio. 16, 100434 (2022).
Wu, Z. et al. Schwann cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 531, 559–565 (2020).
Wang, T. et al. Bioprinted constructs that simulate nerve–bone crosstalk to improve microenvironment for bone repair. Bioact. Mater. 27, 377–393 (2023).
Li, W. et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. interfaces 10, 5240–5254 (2018).
Zhang, Y. et al. A tailored bioactive 3D porous poly(lactic-acid)-exosome scaffold with osteo-immunomodulatory and osteogenic differentiation properties. J. Biol. Eng. 16, 22 (2022).
Liu, K. et al. Macrophage-derived exosomes promote bone mesenchymal stem cells towards osteoblastic fate through microRNA-21a-5p. Front. Bioeng. Biotechnol. 9, 801432 (2021).
Liu, A. et al. Macrophage-derived small extracellular vesicles promote biomimetic mineralized collagen-mediated endogenous bone regeneration. Int. J. Oral. Sci. 12, 33 (2020).
Lopez-Verrilli, M. A., Picou, F. & Court, F. A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61, 1795–1806 (2013).
Zhao, J. et al. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res. Ther. 11, 360 (2020).
Jalilian, E. et al. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res. Ther. 13, 425 (2022).
Hervera, A. et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 (2018).
Cerri, F. et al. Peripheral nerve morphogenesis induced by scaffold micropatterning. Biomaterials 35, 4035–4045 (2014).
Ravoor, J., Thangavel, M. & Elsen S, R. Comprehensive review on design and manufacturing of bio-scaffolds for bone reconstruction. ACS Appl. Bio. Mater. 4, 8129–8158 (2021).
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010).
Liu, Z. et al. Low-stiffness hydrogels promote peripheral nerve regeneration through the rapid release of exosomes. Front. Bioeng. Biotechnol. 10, 922570 (2022).
Rosso, G. et al. Matrix stiffness mechanosensing modulates the expression and distribution of transcription factors in Schwann cells. Bioeng. Transl. Med. 7, e10257 (2022).
Saha, K. et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 (2008).
Pathak, M. M. et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 111, 16148–16153 (2014).
Liang, Y. et al. Impact of hydrogel stiffness on the induced neural stem cells modulation. Ann. Transl. Med. 9, 1784 (2021).
Brunetti, V. et al. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 107, 6264–6269 (2010).
Luo, J. et al. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix - A review. Bioact. Mater. 15, 145–159 (2022).
Jia, Y. et al. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 83, 291–301 (2019).
Dong, X. et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 272, 120767 (2021).
Dong, X. et al. Oriented nanofibrous P(MMD-co-LA)/Deferoxamine nerve scaffold facilitates peripheral nerve regeneration by regulating macrophage phenotype and revascularization. Biomaterials 280, 121288 (2022).
Hu, X. et al. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng. Part A 15, 3297–3308 (2009).
Milos, F., Belu, A., Mayer, D., Maybeck, V. & Offenhäusser, A. Polymer nanopillars induce increased paxillin adhesion assembly and promote axon growth in primary cortical neurons. Adv. Biol. 5, 2000248 (2021).
Li, Q. et al. Micropatterned photothermal double-layer periosteum with angiogenesis-neurogenesis coupling effect for bone regeneration. Mater. Today Bio. 18, 100536 (2023).
Johansson, F., Kanje, M., Eriksson, C. & Wallman, L. Guidance of neurons on porous patterned silicon: is pore size important. Phys. Status Solidi (c.) 2, 3258–3262 (2005).
Gentile, F. et al. Differential cell adhesion on mesoporous silicon substrates. ACS Appl. Mater. interfaces 4, 2903–2911 (2012).
Li, Y., Hoffman, M. D. & Benoit, D. Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature. Biomaterials 268, 120535 (2021).
Zhang, M. et al. 3D printing of tree-like scaffolds for innervated bone regeneration. Addit. Manuf. 54, 102721 (2022).
Zhang, M. et al. 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 6, eaaz6725 (2020).
Wang, X. et al. Mimicking bone matrix through coaxial electrospinning of core-shell nanofibrous scaffold for improving neurogenesis bone regeneration. Biomater. Adv. 145, 213246 (2023).
Zhang, Z. et al. Engineered sensory nerve guides self-adaptive bone healing via NGF-TrkA signaling pathway. Adv. Sci. (Weinh), e2206155 (2023).
Jimbo, R. et al. The effect of brain-derived neurotrophic factor on periodontal furcation defects. PLoS One 9, e84845 (2014).
Jimbo, R. et al. Regeneration of the cementum and periodontal ligament using local BDNF delivery in class II furcation defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 106, 1611–1617 (2018).
Ramalho, I. et al. Periodontal tissue regeneration using brain-derived neurotrophic factor delivered by collagen sponge. Tissue Eng. Part A 25, 1072–1083 (2019).
Liao, J. et al. Peptide-modified bone repair materials: factors influencing osteogenic activity. J. Biomed. Mater. Res. A 107, 1491–1512 (2019).
Liang, T. et al. Drug-loading three-dimensional scaffolds based on hydroxyapatite-sodium alginate for bone regeneration. J. Biomed. Mater. Res. A 109, 219–231 (2021).
Wallach, S. Effects of magnesium on skeletal metabolism. Magn. Trace Elem. 9, 1–14 (1990).
Baker, A. et al. Effect of dietary copper intakes on biochemical markers of bone metabolism in healthy adult males. Eur. J. Clin. Nutr. 53, 408–412 (1999).
Bari, A. et al. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 55, 493–504 (2017).
Liu, Y. et al. Biodegradable metal-derived magnesium and sodium enhances bone regeneration by angiogenesis aided osteogenesis and regulated biological apatite formation. Chem. Eng. J. 410, 127616 (2021).
Zhao, Y. et al. Construction of macroporous magnesium phosphate-based bone cement with sustained drug release. Mater. Des. 200, 109466 (2021).
Chakraborty Banerjee, P., Al-Saadi, S., Choudhary, L., Harandi, S. E. & Singh, R. Magnesium implants: prospects and challenges. Materials (Basel), (2019).
Zhang, D. et al. Targeting local osteogenic and ancillary cells by mechanobiologically optimized magnesium scaffolds for orbital bone reconstruction in canines. ACS Appl. Mater. interfaces 12, 27889–27904 (2020).
Li, W. et al. Bioprinted constructs that mimic the ossification center microenvironment for targeted innervation in bone regeneration. Adv. Funct. Mater. 32, 2109871 (2022).
Götz, W., Tobiasch, E., Witzleben, S. & Schulze, M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics. 11(2019).
Zhou, X., Zhang, N., Mankoci, S. & Sahai, N. Silicates in orthopedics and bone tissue engineering materials. J. Biomed. Mater. Res. A 105, 2090–2102 (2017).
Ma, Y. X. et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioact. Mater. 9, 475–490 (2022).
Khare, D., Basu, B. & Dubey, A. K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 258, 120280 (2020).
Marsudi, M. A. et al. Conductive polymeric-based electroactive scaffolds for tissue engineering applications: current progress and challenges from biomaterials and manufacturing perspectives. Int. J. Mol. Sci. 22(2021).
Xia, F., Wang, H., Hwang, J. C. M., Neto, A. H. C. & Yang, L. Black phosphorus and its isoelectronic materials. Nat. Rev. Physics 1, 306–317 (2019).
Jing, X., Xiong, Z., Lin, Z. & Sun, T. The application of black phosphorus nanomaterials in bone tissue engineering. Pharmaceutics. 14(2022).
Su, Y. et al. Endogenous electric field-coupled PD@BP biomimetic periosteum promotes bone regeneration through sensory nerve via fanconi anemia signaling pathway. Adv. Healthc. Mater. e2203027 (2023).
Xu, C. et al. Black‐phosphorus‐incorporated hydrogel as a conductive and biodegradable platform for enhancement of the neural differentiation of mesenchymal stem cells. Adv. Funct. Mater. 30, 2000177 (2020).
Li, S. et al. Injectable thermosensitive black phosphorus nanosheet- and doxorubicin-loaded hydrogel for synergistic bone tumor photothermal-chemotherapy and osteogenesis enhancement. Int. J. Biol. Macromol. 239, 124209 (2023).
Ma, S. et al. Calcium phosphate bone cements incorporated with black phosphorus nanosheets enhanced osteogenesis. ACS Biomater. Sci. Eng. 9, 292–302 (2023).
Borges, M. et al. Recent advances of polypyrrole conducting polymer film for biomedical application: Toward a viable platform for cell-microbial interactions. Adv. Colloid Interface Sci. 314, 102860 (2023).
Chen, J., Yu, M., Guo, B., Ma, P. X. & Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 514, 517–527 (2018).
Flores-Sánchez, M. G. et al. Effect of a plasma synthesized polypyrrole coverage on polylactic acid/hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 109, 2199–2211 (2021).
Vishnoi, T., Singh, A., Teotia, A. K. & Kumar, A. Chitosan-gelatin-polypyrrole cryogel matrix for stem cell differentiation into neural lineage and sciatic nerve regeneration in peripheral nerve injury model. ACS Biomater. Sci. Eng. 5, 3007–3021 (2019).
Wu, C. et al. Antioxidative and conductive nanoparticles-embedded cell niche for neural differentiation and spinal cord injury repair. ACS Appl. Mater. Interfaces. (2021).
Beygisangchin, M., Abdul Rashid, S., Shafie, S., Sadrolhosseini, A. R. & Lim, H. N. Preparations, properties, and applications of polyaniline and polyaniline thin films-a review. Polymers (2021).
Wang, Q. et al. Direct current stimulation for improved osteogenesis of MC3T3 cells using mineralized conductive polyaniline. ACS Biomater. Sci. Eng. 7, 852–861 (2021).
Kim, H. J. et al. Fabrication of nanocomposites complexed with gold nanoparticles on polyaniline and application to their nerve regeneration. ACS Appl. Mater. interfaces 12, 30750–30760 (2020).
Deng, P., Chen, F., Zhang, H., Chen, Y. & Zhou, J. Multifunctional double-layer composite hydrogel conduit based on chitosan for peripheral nerve repairing. Adv. Health. Mater. 11, e2200115 (2022).
Eivazzadeh-Keihan, R. et al. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: a review. J. Adv. Res. 18, 185–201 (2019).
Li, Y. et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 18, 213–227 (2022).
Zhang, X. et al. 3D printed reduced graphene oxide-GelMA hybrid hydrogel scaffolds for potential neuralized bone regeneration. J. Mater. Chem. B 11, 1288–1301 (2023).
Zhao, Y. N. et al. Electrodeposition of chitosan/graphene oxide conduit to enhance peripheral nerve regeneration. Neural Regen. Res. 18, 207–212 (2023).
Pi, W. et al. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication. 14(2022).
Cao, Y. et al. 3D printed-electrospun PCL/hydroxyapatite/MWCNTs scaffolds for the repair of subchondral bone. Regen. Biomater. 10, rbac104 (2023).
Battiston, K. G., Cheung, J. W., Jain, D. & Santerre, J. P. Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials 35, 4465–4476 (2014).
Yoo, J., Jung, Y., Char, K. & Jang, Y. Advances in cell coculture membranes recapitulating in vivo microenvironments. Trends Biotechnol. 41, 214–227 (2023).
Xu, Y. et al. A silk fibroin/collagen nerve scaffold seeded with a co-culture of schwann cells and adipose-derived stem cells for sciatic nerve regeneration. PLoS One 11, e0147184 (2016).
Resch, A. et al. Co-culturing human adipose derived stem cells and schwann cells on spider silk-a new approach as prerequisite for enhanced nerve regeneration. Int. J. Mol. Sci. 20(2018).
Zheng, T. et al. Co-culture of Schwann cells and endothelial cells for synergistically regulating dorsal root ganglion behavior on chitosan-based anisotropic topology for peripheral nerve regeneration. Burns Trauma. 10, tkac030 (2022).
Li, Y., Men, Y., Wang, B., Chen, X. & Yu, Z. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J. Mater. Sci. Mater. Med. 31, 99 (2020).
Santos, M. I., Unger, R. E., Sousa, R. A., Reis, R. L. & Kirkpatrick, C. J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 30, 4407–4415 (2009).
Chen, W. et al. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 12, 191–203 (2018).
Carvalho, M. S. et al. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C. Mater. Biol. Appl. 99, 479–490 (2019).
Yang, Y. et al. Magnesium-based whitlockite bone mineral promotes neural and osteogenic activities. ACS Biomater. Sci. Eng. 6, 5785–5796 (2020).
Iaquinta, M. R. et al. Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 7, 268 (2019).
Liu, Y., Chan, J. K. & Teoh, S. H. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regen. Med. 9, 85–105 (2015).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Jiang, Y. et al. Neuronal TRPV1-CGRP axis regulates bone defect repair through Hippo signaling pathway. Cell. Signal. 109, 110779 (2023).
Ferrigno, B. et al. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 5, 468–485 (2020).
Khalifeh, J. M. et al. Electrical stimulation and bone Healing: A review of current technology and clinical applications. IEEE Rev. Biomed. Eng. 11, 217–232 (2018).
Wang, J., Wang, H., Mo, X. & Wang, H. Reduced graphene oxide-encapsulated microfiber patterns enable controllable formation of neuronal-like networks. Adv. Mater. Weinh. 32, e2004555 (2020).
Lau, Y. C., Qian, X., Po, K. T., Li, L. M. & Guo, X. Electrical stimulation at the dorsal root ganglion preserves trabecular bone mass and microarchitecture of the tibia in hindlimb-unloaded rats. Osteoporos. Int. 26, 481–488 (2015).
Jiang, Y. X., Gong, P. & Zhang, L. [A review of mechanisms by which low-intensity pulsed ultrasound affects bone regeneration]. Hua Xi Kou Qiang Yi Xue Za Zhi 38, 571–575 (2020)..
Yang, M. H., Lim, K. T., Choung, P. H., Cho, C. S. & Chung, J. H. Application of ultrasound stimulation in bone tissue engineering. Int. J. Stem Cells 3, 74–79 (2010).
Jiang, W. et al. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci. Rep. 6, 22773 (2016).
Lam, W. L., Guo, X., Leung, K. S. & Kwong, K. S. The role of the sensory nerve response in ultrasound accelerated fracture repair. J. Bone Jt. Surg. Br. 94, 1433–1438 (2012).
Reher, P., Harris, M., Whiteman, M., Hai, H. K. & Meghji, S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone 31, 236–241 (2002).
Tang, C. H. et al. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol. Pharmacol. 69, 2047–2057 (2006).
McCarthy, C. & Camci-Unal, G. Low intensity pulsed ultrasound for bone tissue engineering. Micromachines (Basel). 12(2021).
Mao, X. et al. Nerve ECM and PLA-PCL based electrospun bilayer nerve conduit for nerve regeneration. Front. Bioeng. Biotechnol. 11, 1103435 (2023).
Wang, X. et al. A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis. Bioact. Mater. 21, 110–128 (2023).
Halperin-Sternfeld, M. et al. Immunomodulatory fibrous hyaluronic acid-Fmoc-diphenylalanine-based hydrogel induces bone regeneration. J. Clin. Periodontol. 50, 200–219 (2023).
Yang, J. et al. A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats. Neural Regen. Res. 18, 657–663 (2023).
Jafarimanesh, M. A. et al. Sustained release of valproic acid loaded on chitosan nanoparticles within hybrid of alginate/chitosan hydrogel with/without stem cells in regeneration of spinal cord injury. Prog Biomater. (2023).
Zheng, A. et al. Promoting lacunar bone regeneration with an injectable hydrogel adaptive to the microenvironment. Bioact. Mater. 21, 403–421 (2023).
Cheng, D. et al. Strontium ion-functionalized nano-hydroxyapatite/chitosan composite microspheres promote osteogenesis and angiogenesis for bone regeneration. ACS Appl. Mater. Interfaces. (2023).
Sow, W. T. et al. Freeze-casted keratin matrix as an organic binder to integrate hydroxyapatite and BMP2 for enhanced cranial bone regeneration. Adv. Healthc. Mater. e2201886 (2022).
Qin, H. J. et al. Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects. Neural Regen. Res. 18, 1118–1123 (2023).
Gao, X. et al. Nerve growth factor-laden anisotropic silk nanofiber hydrogels to regulate neuronal/astroglial differentiation for scarless spinal cord repair. ACS Appl. Mater. interfaces 14, 3701–3715 (2022).
Wang, X. et al. Chitosan/silk fibroin composite bilayer PCL nanofibrous mats for bone regeneration with enhanced antibacterial properties and improved osteogenic potential. Int. J. Biol. Macromol. 230, 123265 (2023).
Ma, J. et al. Collagen modified anisotropic PLA scaffold as a base for peripheral nerve regeneration. Macromol. Biosci. 22, e2200119 (2022).
Li, J. et al. Dual-Nozzle 3D printed nano-hydroxyapatite scaffold loaded with vancomycin sustained-release microspheres for enhancing bone regeneration. Int. J. Nanomed. 18, 307–322 (2023).
Manto, K. M. et al. Erythropoietin-PLGA-PEG as a local treatment to promote functional recovery and neurovascular regeneration after peripheral nerve injury. J. Nanobiotechnol. 20, 461 (2022).
Wei, J. et al. Sequential dual-biofactor release from the scaffold of mesoporous HA microspheres and PLGA matrix for boosting endogenous bone regeneration. Adv Healthc. Mater. e2300624 (2023).
Yao, Z. et al. Magnesium-encapsulated injectable hydrogel and 3D-engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv. Sci. (Weinh.) 9, e2202102 (2022).
Kang, D. et al. FeS2-incorporated 3D PCL scaffold improves new bone formation and neovascularization in a rat calvarial defect model. Int. J. Bioprint 9, 636 (2023).
Zhang, S. et al. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater. 134, 116–130 (2021).
Xia, Y. et al. 3D-printed dual-ion chronological release functional platform reconstructs neuro-vascularization network for critical-sized bone defect regeneration. Chem. Eng. J. 465, 143015 (2023).
Li, R. X. et al. Highly bioactive peptide-HA photo-crosslinking hydrogel for sustained promoting bone regeneration. Chem. Eng. J. 415, 129015 (2021).
Jiang, Y. et al. Low-intensity pulsed ultrasound improves osseointegration of dental implant in mice by inducing local neuronal production of αCGRP. Arch. Oral. Biol. 115, 104736 (2020).
Liu, X., Zou, D., Hu, Y., He, Y. & Lu, J. Research progress of low-intensity pulsed ultrasound in the repair of peripheral nerve injury. Tissue Eng. Part B Rev. (2023).
Ye, J., Huang, B. & Gong, P. Nerve growth factor-chondroitin sulfate/hydroxyapatite-coating composite implant induces early osseointegration and nerve regeneration of peri-implant tissues in Beagle dogs. J. Orthop. Surg. Res. 16, 51 (2021).
Eap, S. et al. Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth. Adv. Health. Mater. 3, 386–391 (2014).
Ye, J. & Gong, P. NGF-CS/HA-coating composite titanium facilitates the differentiation of bone marrow mesenchymal stem cells into osteoblast and neural cells. Biochem. Biophys. Res. Commun. 531, 290–296 (2020).
Kauschke, V. et al. Effects of a pasty bone cement containing brain-derived neurotrophic factor-functionalized mesoporous bioactive glass particles on metaphyseal healing in a new murine osteoporotic fracture model. Int. J. Mol. Sci. 19(2018).
Takeda, K. et al. Characteristics of high-molecular-weight hyaluronic acid as a brain-derived neurotrophic factor scaffold in periodontal tissue regeneration. Tissue Eng. Part A 17, 955–967 (2011).
Li, Y. et al. Bio-Oss® modified by calcitonin gene-related peptide promotes osteogenesis in vitro. Exp. Ther. Med. 14, 4001–4008 (2017).
Yu, X. et al. CGRP gene-modified rBMSCs show better osteogenic differentiation capacity in vitro. J. Mol. Histol. 49, 357–367 (2018).
Yu, X. et al. Calcitonin gene related peptide gene-modified rat bone mesenchymal stem cells are effective seed cells in tissue engineering to repair skull defects. Histol. Histopathol. 34, 1229–1241 (2019).
Lv, T. et al. Novel calcitonin gene-related peptide/chitosan-strontium-calcium phosphate cement: Enhanced proliferation of human umbilical vein endothelial cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 19–28 (2019).
Luo, J. et al. CGRP-loaded porous microspheres protect BMSCs for alveolar bone regeneration in the periodontitis microenvironment. Adv. Healthc. Mater. 12, e2301366 (2023).
Chen, J. et al. Gelatin microspheres containing calcitonin gene-related peptide or substance P repair bone defects in osteoporotic rabbits. Biotechnol. Lett. 39, 465–472 (2017).
Lotz, E. M., Berger, M. B., Boyan, B. D. & Schwartz, Z. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 134, 115260 (2020).
Kim, S. H., Kim, J. E., Kim, S. H. & Jung, Y. Substance P/dexamethasone-encapsulated PLGA scaffold fabricated using supercritical fluid process for calvarial bone regeneration. J. Tissue Eng. Regen. Med. 11, 3469–3480 (2017).
Amirthalingam, S. et al. Addition of lactoferrin and substance P in a chitin/PLGA-CaSO4 hydrogel for regeneration of calvarial bone defects. Mater. Sci. Eng. C. Mater. Biol. Appl. 126, 112172 (2021).
Mu, C. et al. Substance P-embedded multilayer on titanium substrates promotes local osseointegration via MSC recruitment. J. Mater. Chem. B 8, 1212–1222 (2020).
Chen, D. et al. Nacre-mimetic hydroxyapatite/chitosan/gelatin layered scaffolds modifying substance P for subchondral bone regeneration. Carbohydr. Polym. 291, 119575 (2022).
Noh, S. S. et al. A dual delivery of substance P and bone morphogenetic protein-2 for mesenchymal stem cell recruitment and bone regeneration. Tissue Eng. Part A 21, 1275–1287 (2015).
Wang, T. et al. Substance P incorporation in calcium phosphate cement for dental alveolar bone defect restoration. Mater. Sci. Eng. C. Mater. Biol. Appl. 69, 546–553 (2016).
La, W. G. et al. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 9, 107–116 (2014).
Kim, S. H. et al. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng. Part A 21, 1237–1246 (2015).
Ji, H. et al. Programmed core-shell electrospun nanofibers to sequentially regulate osteogenesis-osteoclastogenesis balance for promoting immediate implant osseointegration. Acta Biomater. 135, 274–288 (2021).
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 82072446, 81873999, 82272460, 82202715, 82102546, 82102627) and the Natural Science Foundation of Hubei Province (2021CFB277, 2021CFB596).
Author information
Authors and Affiliations
Contributions
W.S., B.Y., and S.C. conceived the review; W.S. and S.C. defined the intellectual content; L.Z., Y.W., S.C., Q.G., and H.L. performed the literature searches; W.S., B.Y., S.C., L.Z., H.L., and Q.G. acquired the data; W.S., S.C., H.L., K.C., L.Z. and Y.W. performed the data analysis; W.S. prepared and finished the manuscript; W.S., B.Y., Y.Q., and L.Z. edited the manuscript; B.Y., W.S., and S.C. reviewed the manuscript; and W.B., X.L. and X.G. supervised manuscript drafting and determined the final draft. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Permission to reproduce material from other sources
Figures were all created with BioRender.com, and publication licenses were obtained.
Declaration of Generative AI and AI-assisted technologies in the writing process
AI was not used during the preparation of this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, W., Ye, B., Chen, S. et al. Neuro–bone tissue engineering: emerging mechanisms, potential strategies, and current challenges. Bone Res 11, 65 (2023). https://doi.org/10.1038/s41413-023-00302-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41413-023-00302-8