Abstract
The aim of this study was to investigate the control of three species of bacteria commonly associated with biologically contaminated water, using biofiltration. In this study, a laboratory-scale biofilter system was used to investigate the control of Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa in fresh water. Simulated fresh water was inoculated with the test pathogens at a starting inocula of ~1000 CFU 100 mL−1 to challenge the biofilters. Biofilter systems operating within a recirculation configuration demonstrated significant reduction of E. coli (99%), E. faecalis (99%), and P. aeruginosa (92%) after 24 h. Conversely, all sterile control systems did not show any significant reduction in pathogens. Subsequent analysis of the biofilter media after circulation showed that 0% of E. coli was recovered from the biofilter, whereas 0.06% and 1.26% of E. faecalis and P. aeruginosa were recovered respectively. Further investigation demonstrated the reduction of E. coli and enterococci from an environmentally-derived surface water of 99.8% and 99.4% respectively. In conclusion, this work demonstrates that biofilter systems can be used to significantly reduce waterborne pathogenic bacteria within fresh water. The potential application of low-cost, energy efficient biofilter systems for the management of waterborne bacterial pathogens in water supplies is discussed.
Similar content being viewed by others
Introduction
Meeting the future demands for fresh water has been identified as a major global challenge over the coming decades1,2. Of all water present on Earth, only 2.5% is fresh water. Furthermore, only 0.77% of fresh water is deemed accessible, and of this, only 10% is reported to be suitable for human consumption3,4. Since 2000, billions of people have gained access to basic drinking water and sanitation services, however, access to safe water free from chemical and biological contamination (potable water) remains a significant challenge5. Water that is contaminated with microorganisms, can cause disease; the consumption of biologically contaminated water is estimated to cause 485,000 deaths each year6. Such diseases can be attributed to the presence of pathogenic bacteria, including, but not limited to, Escherichia coli, enterococci species (including Enterococcus faecalis) and Vibrio cholera within untreated water7,8,9,10. Nonetheless, biology plays an important role in access to improved drinking water. Slow sand filters are commonly used in low-middle income countries (LMIC). The development of a layer of biomass (schmutzdecke) at the surface of the sand filter facilitates the biodegradation of organic material present in the water. This biomass layer also acts to prevent the physical progression of waterborne pathogens such as E. coli, coliform bacteria, Giardia and Cryptosporidium11,12,13 through the slow sand filter, commonly achieving between 1 and 4 log reductions in pathogen loads. In high income countries, the biological filters are commonly used to reduce or remove organic matter, a precursor to the formation of harmful disinfection by products, prior to chlorination14,15,16. In addition, biological filtration methods are commonly used for the treatment (tertiary stage) of municipal wastewater17.
The control of waterborne pathogenic bacteria in areas with established, centralised water treatment systems is achieved by the combination of physical processes such as screening and filtration with well understood disinfection methods using chemicals, ozonation and/or UV disinfection18, which require significant energy and resources. Moreover, chlorination is used in drinking water disinfection processes, requiring residual free chlorine and chloramines throughout distribution systems to ensure water quality is maintained, preventing the growth of pathogenic bacteria during water distribution19. However, such disinfection agents are hazardous, expensive, and not always easily implemented in remote or challenging communities where infrastructure is lacking. Therefore, there is an unmet need for low energy solutions for the provision of potable water that are sustainable in the long term, i.e. scalable with low energy, maintenance and material requirements.
Over the last 20 years, there has been an increase into the research and development of sustainable, compact water treatment systems for the production of drinking water (Table 1)20. Sustainable provision of potable water should provide adequate water quantity and appropriate water quality for a given need, without compromising the future ability to provide this capacity and quality. A few examples of this are in ground water stores such as aquifers where the demand is no more than the recharge rate, recycled/reclaimed water, and rainwater harvesting. Appropriate treatment methods will also contribute to water sustainability, whereby the maintenance of appropriate water quality can reduce pressure on this resource. One potential sustainable approach for water treatment is the application of biofiltration, a remediation biotechnology that utilises microbial biofilms adhered to a stationary phase substrate that can be configured within filter columns15. Biofilms are complex microbial communities and can be defined as aggregates of microorganisms in which cells are frequently embedded in a self-produced matrix of extracellular polymeric substances (EPS). The production of EPS facilitates the adherence of the microorganisms to each other as well as to biotic and abiotic surfaces21. Biofiltration utilises granular filter media upon which biofilms can establish. The types of filter media employed are varied and can be comprised of sand, polymer, ceramic, charcoal and granular activated carbon, amongst others15.
Where biofilters are used in water treatment, the biofilm communities that are established on filter media (biofilter media) are known to be diverse22,23,24. Such biofilters are used to reduce levels of natural organic material in drinking water treatment22 or the biochemical oxygen demand of wastewaters25. These diverse microbial populations exhibit complex sorbent characteristics; whereby binding sites within microbial biofilm communities include both anionic and cationic exchangers that can remove a wide range of substances and contaminants from the water source, even when such contaminants are present at very low concentrations26,27,28. Nutrient acquisition is an essential driver for this process, and biofilms have developed a very efficient capture strategy for this. The sorption properties of the EPS matrix enable it to behave like a sponge that influences the exchange of nutrients, gases and other molecules between the water phase and the biofilm21. Moreover, a diverse biofilm community will incorporate varying sorption mechanisms and binding sites of biofilm cells and the EPS of the matrix and can apply to both dissolved compounds and suspended solids. These compounds can be trapped by biofilms and incorporated into the matrix, including biodegradable material that can be utilised as a source of nutrients for further microbial growth. The capability of biofilms to remove a wide range of substances such as nutrients involve cooperation. For example, in the process of nitrification ammonia-oxidising bacteria metabolise ammonia into nitrite which is then utilised by other nitrite oxidising species of bacteria such as Nitrospira moscoviensis. Moreover, aerobic bacteria consume oxygen which can exceed the rate of diffusion through the biofilm, facilitating the survival of anaerobic bacteria such as those involved in denitrification of nitrates29. Moreover, metal ions including but not limited to Cu2+ Zn2+, Fe2+, Fe3+ and Al3+ have been found to accumulate within biofilms30. Although, biofilters have been shown to remove a wide range of chemical substances24,31,32, little is known regarding the use of biofilters for the control and removal of pathogenic bacteria in potable water supplies. For example, biofiltration has been demonstrated to result in a reduction of E. coli (up to 58%) and faecal coliforms (up to 63%) in urban ponds33. For potable water production, a combination of biofiltration technology, coagulation, flocculation, filtration and chlorination has been previously demonstrated to be effective for the control of waterborne bacteria34. However, to date no studies have been reported that demonstrate the use of biofiltration alone to control the levels of pathogenic bacteria for potable water. The aim of this study was to investigate the control and inhibition of pathogenic bacteria commonly associated with biologically contaminated water supplies using biofiltration.
Results
Viable counts of Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa are significantly reduced by biofiltration
At the start of all experiments, there was no significant difference in the starting density of test pathogen monocultures within the simulated freshwater (SFW), between the biofilter, the column containing the sterile filter media and the empty filter column (p > 0.05; Fig. 1). After 24 h, significant reductions in all three species were observed in the biofilter systems, when compared to the SFW in both the control systems (p < 0.001; Fig. 1a–c). Relative to the initial bacterial load present within the SFW, the biofilter was shown to significantly reduce E. coli cell numbers by 99.4 ± 0.60% (absolute mean reduction by 4.02 ± 0.01 Log10 CFU; Fig. 1a), E. faecalis by 99.9 ± 0.04% (absolute mean reduction by 4.01 ± 0.22 Log10 CFU; Fig. 1b), and P. aeruginosa by 92.1 ± 10.9% (absolute mean reduction by 3.93 ± 0.093 Log10 CFU; Fig. 1c). The control system containing sterile filter media also resulted in significant reductions for E. coli and E. faecalis, whereby a reduction from 3.97 ± 0.06 Log10CFU to 3.48 ± 0.07 Log10CFU for E. coli and 3.91 ± 0.15 Log10CFU to 3.64 ± 0.14 Log10CFU for E. faecalis was observed. However, an increase from 4.01 ± 0.04 Log10CFU to 6.55 ± 1.09 Log10CFU was observed for P. aeruginosa. For the system with the empty filter column small non-significant reductions were observed for E. coli and E. faecalis (p > 0.05 Fig. 1a, b). There was a reduction from 3.94 ± 0.02 to 3.82 ± 0.08 Log10CFU for E. coli and a reduction from 3.91 ± 0.14 to 3.77 ± 0.15 Log10CFU for E. faecalis. However, there was increase to from 3.93 ± 0.13 to 6.69 ± 0.94 Log10CFU for P. aeruginosa in the system with the empty filter column.
Viable counts of a Escherichia coli, b Enterococcus faecalis and c Pseudomonas aeruginosa within simulated freshwater when circulated through a laboratory-scale system incorporating a biofilter, a column containing the sterile filter media and an empty filter column over 24 h, all performed in triplicate. Statistical significance determined using two-way ANOVA with Tukey’s post-hoc test from a minimum of n = 3 ± s.d. (***=p < 0.001).
The accumulation of Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa on the filter media
For E. coli, no viable cells were recovered from the biofilter media, whereas 3.03 ± 0.03 Log10CFU filter−1 was recovered from the sterile filter media (Table 2). For E. faecalis, 0.91 ± 0.65 Log10CFU filter−1 was recovered from the biofilter after the complete 24 h cycle. This equates to 0.06% of the starting density that was able to accumulate and survive on the biofilter media after 24 h, however, this was significantly less than the 2.46 ± 0.14 Log10CFU filter−1 recovered from the sterile filter media (p < 0.05; Table 2). For P. aeruginosa, 1.89 ± 0.99 Log10CFU filter−1 (equating to 1.26% of the starting inoculum) was recovered from the biofilter media. Whereas, 4.24 ± 0.49 Log10CFU filter−1 was recovered from the sterile filter media. Moreover, there was an average increase from 3.93 ± 0.13 Log10CFU to 6.53 ± 1.12 Log10CFU within the system containing sterile filter media in comparison to an average decrease from 3.97 ± 0.04 Log10CFU to 1.89 ± 0.99 Log10CFU for P. aeruginosa when cycled through the biofilter. To ensure that there was no significant difference between the system containing the sterile filter media and the system containing the empty filter column for all three species of bacteria after 24 h of circulation, the total number of cells on the sterile filter media and in the SFW were compared against the number of cells in the SFW of the system with the empty filter column and were found to be non-significant for all three species (p > 0.05).
The circulation of environmental water through biological filters
A range of standard biological and physiochemical indicators of water quality were recorded before and after 24 h of circulation through the test systems. After 24 h, significant reductions in the biological indicator species (E. coli and enterococci) were observed. There was a reduction of 99.8 ± 0.20% and 99.4 ± 0.41% for E. coli and enterococci respectively. There was no significant change in the heterotrophic plate counts in the environmental water post 24 h circulation through the biofilter, however, there was a significant increase of 403% (equating to a total increase of 4.98 Log10CFU mL−1) in heterotrophic plate counts in the environmental water when circulated through the empty filter column.
The concentration of nitrogen species in the environmental water decreased after 24 h of circulation through the biofilter. There was a significant reduction in the concentrations of 100% of ammonium, 100% of nitrite and 30.8 ± 11.3% of nitrate (p < 0.05; Table 3). The 100% reduction of both ammonium and nitrite were recorded as below the detection limit of the ion chromatography. Moreover, the reduction of all three nitrogen species were significantly different from the empty filter column at 24 h. The concentration of nitrogen in the environmental water that was circulated through the empty filter column showed an increase in nitrite of 71% and a small change in the concentration of ammonium and nitrate (p > 0.05; Table 3). The concentration of total inorganic carbon (TIC) increased after 24 h of circulation through the biofilter. There was a significant difference between the biofilter and the empty filter column with the TIC increasing by 5.8% (p < 0.05; Table 3). However, the concentration of total organic carbon (TOC) was not significantly different from the empty filter column (p < 0.05; Table 3). When comparing the water quality parameters of the environmental water pre and post circulation for both the biofilter and empty filter column, there was a significant reduction in ORP (p < 0.01; Table 3), a significant increase in dissolved oxygen (DO) (p < 0.05; Table 3) and no significant difference in conductivity or total dissolved solids (TDS) (p > 0.05; Table 3). However, there was no significant difference between the biofilter and the empty filter column post treatment for DO, pH, ORP, conductivity or TDS (p > 0.05; Table 3).
Discussion
Previous research that has utilised biofilters in water treatment has been primarily focused on the removal of organic material and emerging contaminants22,23,31,32,35,36,37,38,39. The use of biofilters to control waterborne pathogens for drinking water treatment has not been extensively reported in the literature. One previous study demonstrated that biofilters were capable of reducing viable E. coli by 56% through treating contaminated environmental water with gravity fed, open rock biofilter systems33. In addition, a biofilter system that utilised foam to establish a biofilm has been shown to significantly reduce the model non-pathogenic bacterial species, Raoultella terrigena, in comparison to a sterile control system34. Although, this was achieved using a complex multi-step filtration system with the role of the biofilter component in the removal of R. terrigena remaining unclear. Therefore there is limited knowledge of the direct effect of biofilm within small-scale biofilter systems (i.e. in the absence of other treatment interventions), to control the numbers of pathogenic bacteria for potable water. In this study, we demonstrate that laboratory-scale biofilters significantly reduced the number of waterborne pathogenic bacteria within a SFW medium and from an environmentally-derived water source.
The significant reduction in the number of viable test pathogens within the SFW after 24 h of circulation through the biofilter systems (Fig. 1; p < 0.001) may have resulted from cell death that either occurred in part, or in whole, within the SFW or on the biofilter media. To account for any loss of pathogens within the SFW, or through physical filtration effects from the ceramic filter media, control columns consisting of an empty filter column and column containing sterile filter media were employed in parallel with the biofilter. Within the systems incorporating the empty filter column and column containing sterile filter media, there was survival of E. coli and E. faecalis (Fig. 1a, b) and proliferation of P. aeruginosa (Fig. 1c) within the reservoir. This demonstrates that the SFW was able to support the survival of test pathogens and that there was a minimal physical filtration effect from the ceramic filter media over the 24 hour circulation period. Therefore, this strongly suggests that the biofilm present on the filter media was primarily responsible for the reduction of the three test species observed (Fig. 1). One of the possible mechanisms for this significant reduction may have been through the direct competition between the planktonic cells and the biofilm established on the filter media. To support this, the accumulation of test pathogens on the filter media was investigated because of the potential for the biofilter to become a reservoir for pathogenic bacteria. Previous studies have demonstrated that planktonic bacteria have greater affinity for attachment to mature biofilms, as opposed to sterile surfaces40,41,42. Moreover, the formation of biofilm is a key survival strategy for microorganisms in challenging environments and therefore, there is a risk that the planktonic pathogens would survive within the biofilm present on the filter media and contaminate the water supply. Nonetheless, this study demonstrates that viable E. coli was unable to accumulate on the biofilter media whereas, this has been shown in previous studies43. This may have resulted from classical competitive exclusion within the biofilter, whereby the challenging pathogens could have been inhibited by the autochthonous bacteria established within the biofilm present on the biofilter media44. Different competition strategies target bacterial ability to form a biofilm, whereas others can be less specific, resulting in death or the limitation of growth in the competing bacteria44 The competition between cells in biofilms can involve inhibitory or cidal mechanisms, such as the production of antibiotics and bacteriocins45,46 or strategies that compromise growth, such as nutrient depletion47. Surface-active compounds (SACs) could also be produced by the microbial biofilm community (biosurfactants), which are amphipathic lipid-based molecules that lower interfacial tension and some of these biosurfactants display antimicrobial properties48. Therefore, production of these biosurfactants could reduce the ability of the test pathogens to accumulate and establish within the biofilm formed on the biofilter. In addition, interference mechanisms might also be upregulated in response to the presence of competition in the surroundings, known as the competition-sensing hypothesis49.
Overall, there were significant reductions observed for all of the test pathogens (Fig. 1; p < 0.001 and Table 2; p < 0.05). However, there were differences in the reductions of test pathogens in the reservoir and on the filter media. Within the reservoir, the total bacterial reductions observed were 99.4 ± 0.60% for E. coli, and 99.9 ± 0.04% for E. faecalis and 92.1 ± 10.9% for P. aeruginosa. This may result from the differential tolerance of these microorganisms to persist within the simulated freshwater environment. The lower reduction of P. aeruginosa is unsurprising, given it is a ubiquitous environmental bacterium which can survive in oligotrophic environments; including up to 5 years in bottled water50. On the filter media, some viable E. faecalis and P. aeruginosa were recovered from the biofilter media, albeit in very low numbers (Table 2). The inability of E. coli to colonise the biofilter media could possibly be related to the lack of an appropriate ‘colonising partner’, which has been shown within capillary flow cell systems, whereby P. aeruginosa was able to form biofilm as a single coloniser40. Nonetheless, in the absence of biofilm, all of the test pathogens were able to adhere to and survive upon the sterile filter media in significantly higher numbers (Table 2; p < 0.05). Therefore, this provides further evidence of competitive exclusion involving potential inhibitory effects by the biofilm on the test pathogens that accumulated on the filter media within the biofilter systems.
The action of competitive exclusion within the biofilter may also explain why only a small number of P. aeruginosa were recovered from the biofilter media (Table 2), even though an overall mean reduction of 92 ± 10.9% was observed within the SFW (Fig. 2c). This indicates that, at the very least, a small sub-population of the P. aeruginosa used to challenge the biofilters was able to survive within the system after 24 h. Nonetheless, it was found that significantly higher numbers of P. aeruginosa were able to attach and survive on the sterile filter media (4.24 ± 0.49 Log10CFU filter−1) when compared to the biofilter media (2.05 ± 0.11 Log10CFU filter−1) (p < 0.05; Table 2). Moreover, there was a stark increase of P. aeruginosa within the SFW of the systems containing the sterile filter media and the empty filter column to 6.55 ± 1.09 Log10CFU and 6.69 ± 0.94 Log10CFU respectively, in comparison to the mean reduction of P. aeruginosa in the biofilter system to 1.89 ± 0.99 Log10CFU. Collectively, this data demonstrates that the survival of P. aeruginosa is significantly lower within the biofilter systems when compared to the sterile filter media and empty filter column controls (p < 0.001; Table 2). Overall, there was a reduction in the survival of E. coli, E. faecalis and P. aeruginosa within biofilms present on the biofilter media after 24 h of circulation, whereby the introduced test pathogens may have been unable to survive as a result of competitive exclusion by the established biofilm on the filter media.
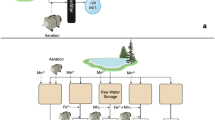
Arrows refer to water flow direction. a Liquid media bottle containing a simulated fresh water (1 L); b Peristaltic pump (35 mL min−1); c Biofilter column containing the ceramic filter media on which a biofilm was established and d a scanning electron micrograph of the environmental biofilm established on the filter media (scale bar 5 µm).
This study demonstrates that matured biofilms present on a ceramic substrate can reduce the numbers of viable E. coli, E. faecalis and P. aeruginosa within a water source used to challenge these biofilters. To further support the reduction of these test pathogens using biofilters, the survival of environmental E. coli and enterococci present within an environmental water source circulated through the lab-scale biofilters was investigated. Biofiltration relies on the processing and metabolism of cells within the biofilm, therefore variability of physiochemical conditions will impact biofiltration in real-world applications. For example, temperature has been shown to directly affect the biodegradation of organics whereby filters exhibit lower microbiological activity at lower temperatures51,52. Nutrient concentrations within the input water to the biofilters have also been shown to have significant impact on performance, whereby the removal of carbon is dependent on the availability of other nutrients such as nitrogen and phosphorous53. Therefore, it was key to investigate the reduction of bacterial pathogens within a water source that was more applicable to biofilter operation in the real-world. This demonstrated that the biofilters are able to reduce the levels of faecal indicator species (potential pathogens) from an environmentally-derived water source (p < 0.05; Table 3). Moreover, the biofilters were able to suppress the growth of heterotrophic bacteria in the environmentally derived water, whereby there was no significant change after 24 h of circulation. However, a significant increase of 4.97 ± 0.14 Log10CFU mL−1 was observed when circulated through the empty filter column (p < 0.05; Table 3). A similar trend was observed when P. aeruginosa was used to challenge the biofilter, whereby a reduction was observed when circulated through the biofilter but an increase of 3.69 ± 0.94 Log10CFU mL−1 was observed in the SFW when circulated through the empty filter column (Fig. 1). P. aeruginosa is ubiquitous in the environment, and is known for its phenotypic variability and ability to actively grow within aquatic freshwater systems53. Therefore, it is likely that the P. aeruginosa used in this study was able to survive and grow within the environmentally-derived water. Moreover, it is possible that the reduction in P. aeruginosa resulted from a phenotypic switch to a viable but non-culturable (VBNC) state where metabolism is downregulated; however, this is more likely when P. aeruginosa is established within biofilms rather than in a planktonic state54. The results from this study indicate that a very small sub-population of P. aeruginosa was able to survive on the biofilter, even though there were still significant reductions of viable P. aeruginosa in the reservoir and on the filter media when compared to the respective controls. The small sub-population of P. aeruginosa that was recovered from the biofilter media after 24 h was significantly less than the sterile filter media. This suggests that the environmental biofilm is exerting some inhibitory effect on planktonic P. aeruginosa.
The significant reduction in the concentration of nitrogen sources within the environmental water (p < 0.05; Table 3) could influence the survival of waterborne pathogens. The nitrification of ammonium is a key part of the global nitrogen cycle that is driven by microorganisms and occurs under aerobic conditions through the following reaction:
Further to this, the removal of nitrate (denitrification) occurs under anoxic conditions through the following reaction:
A metabolic interaction that precedes nitrification can occur when nitrite-oxidizing bacteria supply ammonia to ammonia-oxidizers. These interactions rely on the close proximity of cells that exchange metabolites, to enable efficient exchange by diffusion. The reduction of nitrate within this study indicates possible localised anoxic conditions within the biofilter even though the system was vented, therefore it is likely that oxygen diffusion gradients exist within the biofilm attached to the filter media21. Such localised diffusion gradients of oxygen within biofilms have been previously described, whereby aerobic microbial metabolism can protect anaerobic organisms deeper within the biofilm29,55. Therefore, this could have facilitated the survival of microorganisms that are able to convert nitrate into nitrogen and result in the reductions observed (Table 3). The effect of nutrients including carbon has been shown to impact the growth and survival of planktonic bacteria in water56,57. However, there was no significant reduction in carbon within this study (Table 3; p > 0.05), and hence the reduction of viable indicator species observed is more likely to have occurred through the direct action of the biofilm on the test species. There was no significant difference in TOC between the biofilter and empty filter column control. However, there was a significant difference in TIC after 24 hours of circulation. It is possible that the TOC increased from cell death which was then mineralised by the biofilters to result in the observed increase in TIC. Moreover, denitrification that was observed in this study has also been shown to be involved with the precipitation of TIC44. Therefore, this could be linked with the increase in DIC found in the biofilter systems (Table 3; p < 0.05).
Biofilters in a recirculation configuration can be used to manage water quality by reducing waterborne pathogens in stored water facilities. Such management of pathogens will reduce the risk of illness associated with contaminated water to the end users. Current approaches, such as slow sand filtration, have demonstrated effective reductions in viable pathogens such as E. coli. However, regrowth of E. coli within water storage tanks post slow sand filtration have been identified58,59. The use of recirculating biofiltration systems would offer practical solutions to the management of harvested rainwater, stored ground and surface waters. The use of multiple biofilters configured in series, could significantly scale-up the volumes of water that can be treated. Another practical application of this work is the use of recirculation biofiltration systems, in series, to reduce the biological burden of final effluent discharges. Potentially improving the water quality of designated inland bathing waters or catchments used for drinking water supply.
Overall, this study demonstrates that the process of biofiltration creates an unfavourable aquatic environment for the survival of the planktonic test pathogens and environmental indicator species investigated. However, the specific mechanisms that resulted in a reduction of the viability of waterborne bacterial pathogens are not yet known and further research is necessary. We clearly demonstrate that environmental biofilms significantly reduce the viability of waterborne pathogenic bacteria; however, the following points are critical to further elucidate and optimise this process:
-
1.
Further research into the mechanism of action by which the biofilter is reducing the number of viable waterborne pathogens.
-
2.
Characterisation of the longevity of pathogen removal, including study of potential VBNC bacteria within the system.
-
3.
Investigation of biofilter microbial diversity through functional metagenomics.
-
4.
The upscale of laboratory biofilters to test their performance within a community scale system.
-
5.
Investigation of process parameters, such as contact time, and their impact on pathogen reduction.
The competition between the biofilm established on the filter media and the test pathogens, is a key factor for the reduction observed (Fig. 1 and Table 2). Species groups that are involved in the killing mechanisms within the biofilm could be investigated through the use of functional metagenomics, searching for the potential production of compounds that could inhibit the waterborne bacterial pathogens such as bacteriocins, antibiotics and toxins.
In relation to the removal of organic matter, the function and stability of biofilters has been previously studied through investigating the effect of dosing matured biofilters with nutrients, including ammonium (NH4+) and phosphate (PO4−3)22. These studies concluded that the addition of PO4−3 plays an important role in enhancing the removal of biodegradable dissolved organic carbon. Moreover, it has been shown that in drinking water systems, PO4−3 is the critical limiting factor for limiting or promoting microbial growth60,61. Therefore, the effect of nutrient concentrations on the performance of biofilters in relation to the reduction of waterborne pathogens warrants further investigation. Nutrient deprivation within the biofilter could compromise one of the main mechanisms of pathogen control. Further work is required to investigate this, including the use of upscaled biofilter systems. This would serve to maintain the control of nutrients within the treated water, ultimately enhancing the control of potential pathogens.
The concept of controlling pathogens using biofilters without the aid of chemical or UV disinfection is a challenging one. Even so, this study demonstrates, under laboratory conditions, that biofilters can significantly reduce the levels of E. coli, enterococci (including E. faecalis) and P. aeruginosa within a fresh water source. Currently, for water to be biologically safe, the level of E. coli and enterococci (indicator species) must be 0 CFU 100 mL−1. This study has demonstrated that biofiltration was able to reduce the viability of test pathogens and bacterial indicator species to very low levels, however this would not be classified as potable water. Nonetheless, the reduction of the indicator species observed in this study may be significant to reduce the risk of illness. The global effort into research and development on new approaches of drinking water treatment for developing communities and the remediation of emerging contaminants is crucial to progress towards achieving the 2030 United Nations (UN) Sustainable Development Goals (SDGs)62. This approach has the potential to significantly impact on the delivery of the sustainable development goal number 6: clean water and sanitation for all. Moreover, this would significantly reduce the load on other treatment methods such as UV water clarifiers that consume low levels of energy to reduce the remainder of the indicator species to potable water standards. Therefore, biofilters could be used as the main source of water treatment for low technology solutions for improving access to water free from biological contamination.
Methods
Setup and the maturation of test biofiltration system
The laboratory test biofilters comprised a 500 mL PVC column (63 mm internal diameter, 160 mm length) containing 250 mL of ceramic filter media. The ceramic filter media had a particle size and density of 20 ± 1 mm and 1.16 g mL−1 respectively, and was sterilised by autoclaving before use. The resulting bed porosity was determined using methods outlined by McKie et al.24 and calculated as 2.99. The filter columns containing filter media were connected to circulation tanks (25 L) and were allowed to mature over a period of 4 weeks to establish and achieve mature biofilters. Prior to the maturation process, the circulation tanks were filled with mains tap water and flocculated of residual chlorine via aeration for 24 h (termed recirculation water). Water taken from environmentally-derived surface water held in an urban drainage pond (N 51°29′56″, W 2°32′39″) (250 mL) was then added to inoculate the tanks with a source of environmental microorganisms to provide a seed community for the formation of biofilm on the filter media. At the start of the maturation process, a flow rate of 35 mL min−1 through the filter column was maintained via a multi-channel peristaltic pump (Watson-Marlow 505 S; Falmouth, UK). This ensured that the entire volume of water in the tank was recirculated through the filter column for every 12 h of operation. To further assist the maturation of the biofilm on the filter media, the recirculation water was supplemented with low levels of nutrients on a weekly basis equating to humic acids (50 mg week−1) and NH4CL (5 mg week−1), over the four-week maturation period. The 4-week maturation period was based on experimental demonstration of the stabilisation of biofilm function, quantified by efficient removal of a fixed ammonium load over a 24 h period (in the form of 0.2 mg L−1 ammonium chloride). In addition, for biofilm maturation and preparation, an increase in the number of viable heterotrophic microorganisms present on the filter media after 4 weeks of circulation was also confirmed experimentally. Humic acids were extracted from the soil riparian zone surrounding the aforementioned environmental water source using a method outlined by Serra and Schnitzer.63
Bacterial strains and culture maintenance
The following bacterial strains were chosen because of their relevance as biological indicators of water quality and their potential to cause disease: Escherichia coli NCTC 10418, Enterococcus faecalis NCTC 12679 and Pseudomonas aeruginosa NCIMB 8295. Bacterial strains were isolated and maintained on cryopreservation beads (Microbank, Pro Lab Diagnostics, Canada) at −80 °C, and recovered by plating on nutrient agar (NA; Oxoid™, UK) as required.
Preparation of test pathogen monocultures
Overnight batch cultures of bacterial strains were prepared in 10 mL of nutrient broth (CM0067; Oxoid™, UK) for E. coli and P. aeruginosa and brain heart infusion broth (CM1135; Oxoid™, UK) for E. feacalis and incubated for 16–18 h at 37 °C in an orbital shaker (150 rpm) incubator. Cells were then centrifuged (5 min at 10,000 × g), washed twice and resuspended in 3 L of sterile SFW64.
Bacterial challenge of test biofiltration system
Prior to testing, matured biofilters were disconnected from the circulation tanks, drained and re-connected to a double ported flask (3 × 1 L) containing the test pathogen challenge monoculture (Fig. 2). The test pathogens were circulated through the biofilter at a flow rate of 35 mL min−1. To quantify the reduction of the test pathogens within the SFW, viable counts of E. coli, E. faecalis and P. aeruginosa were recorded over a 24 h duration when individually cycled through the biofilter and control systems. The biofilters were operated in circulation mode to monitor the efficiency of the biofilters to reduce a fixed microbiological load of the test pathogen monoculture and enable the direct comparison between experimental configurations and pathogen species. The SFW was sampled regularly over a 24-hour period; a 10 mL aliquot was taken from the SFW and enumerated using membrane filtration and plating onto selective and differential agar (E. faecalis; Slanetz and Bartley, Oxoid™, Basingstoke, UK. E. coli; Membrane Lactose Glucuronide Agar, Oxoid™, Basingstoke, UK. P. aeruginosa; Pseudomonas C-N Selective Agar, Oxoid™, Basingstoke, UK). A control filter was employed containing sterile ceramic filter media that was pre-soaked with SFW and drained prior to use. An additional control, consisting of an empty 500 mL plastic filter column was used to account for any pathogen reduction resulting from the experimental apparatus. For each of the experimental configurations (empty filter column; column containing sterile filter media; biofilter) three separate experiments were undertaken in parallel. This process was then repeated on three independent occasions (9 filters in total). Within each independent experimental run, all the microbiological sampling (and associated viable counting) was determined in triplicate.
The presence and viability of pathogens on the filter media
To determine if there was any accumulation or retention of the test pathogens on the biofilter media or sterile filter media following the 24 h circulation (Fig. 1), viable counts of E. coli, E. faecalis and P. aeruginosa were taken from the filter media within the filter columns (Fig. 2c). The filter media was removed from the column and suspended in 200 mL of phosphate buffered saline (PBS; Oxoid™, Basingstoke, UK), sonicated for 30 s and vortexed for 30 s (repeated 3 times). Aliquots of the suspension were enumerated using membrane filtration and plated onto selective and differential agar (see section Bacterial challenge of test biofiltration system) to determine the number of test pathogens recovered per filter column.
Environmentally-derived surface water challenge of mature test biofiltration system
To further investigate the control of potential waterborne pathogens under conditions more representative to that found in the environment, environmentally-derived surface water was taken and used (section Setup and the maturation of test biofiltration system) to circulate through the biofilters for 24 h. Following the same preparation procedure outlined in section Bacterial challenge of test biofiltration system, the biofilters were connected to a double ported flask containing water taken from an environmental source known to be contain environmental E. coli and enterococci. Standard biological and physiochemical water quality indicators included in this study: E. coli, enterococci, heterotrophic plate counts (HPCs), ammonium, nitrite, nitrate were taken of the environmental water before and after 24 h circulation. For each of the experimental configurations (empty filter column and biofilters), six separate experiments were operated in parallel. Within each independent experimental run, all the microbiological sampling (and associated viable counting) and the physiochemical parameters were determined in triplicate.
An empty filter column was employed as a control to account for any change in the environmental water that was independent of the biofilter after 24 h of circulation.
Scanning electron microscopy
Established biofilms grown on the filter media were visualised using scanning electron microscopy (SEM). The biofilter media was fixed in 4% glutaraldehyde for 1 h, followed by three 1 h washes in 0.1 M phosphate buffer. Following fixation, the biofilter media was dehydrated by a series of 5 min washes in 30–100% ethanol and then a series of hexamethyldisilane washes with 100% ethanol. Each individual sample was mounted onto a stainless-steel stub, gold splutter coated and inserted into a stub holder on the cooler stage. The SEM (FEI Quanta FEG 650) used the Everhart Thornley detector for all imaging. The biofilm sample was analysed with an acceleration voltage of 5.00 kV and spot size 3.0.
Ion chromatography
Ion Chromatography (Metro HM, 850 Professional IC Anion) was used to quantify the level of anions and cations of relevant nutrients with regards to water quality and microbial growth; phosphate (PO43−), nitrite (NO2−), nitrate (NO3−) and ammonium (NH4+). Samples were taken from the SFW and were filtered through a 0.2 µm filter immediately after collection to prevent any further microbial processing. Aqueous samples were then loaded onto an auto sampler in open top tubes and auto-injected. For anion analysis, a sodium carbonate (3.2 mM L−1) and sodium bicarbonate (1.0 mM L−1) mobile phase was used throughout. Background conductivity of the mobile phase was suppressed by a cation exchanger and regenerated using a dilute sulphuric acid (150 mM L−1) and oxalic acid (100 mM L−1) solution. For cations a nitric acid (0.7 mM L−1) and dipicolonic acid (1.7 mM L−1) mobile phase was used. IC certified standards (Fisher scientific) were used to generate standard curves from which the water samples were interpolated.
Total Carbon analysis
Total Carbon analysis (EnviroTOC, Elementar), was used to quantify the level total inorganic carbon (TIC) and total organic carbon (TOC). Samples were taken from the environmentally-derived surface water and were filtered through a 0.45 µm filter immediately after collection. Aqueous samples were then loaded onto an auto sampler in open top tubes and auto-injected.
Statistical analysis
Statistical analysis of all data was conducted using GraphPad Prism version 9.2.0 for Windows (GraphPad Software, San Diego, CA, USA). T-tests and two-way analysis of variance (ANOVA) with Tukey’s post-hoc test were used where appropriate, with p < 0.05 regarded as significant.
Data availability
All of the data generated and/or analysed during this study is available from the corresponding author upon reasonable request.
References
Holland, R. A. et al. Global impacts of energy demand on the freshwater resources of nations. Proc. Natl Acad. Sci. U.S.A. 112, E6707–E6716 (2015).
Gleick, P. H. & Palaniappan, M. Peak water limits to freshwater withdrawal and use. Proc. Natl Acad. Sci. U.S.A. 107, 11155–11162 (2010).
Oki, T. & Kanae, S. Global hydrological cycles and world water resources. Science. 313, 1068–1072 (2006).
Postel, S. L., Daily, G. C. & Ehrlich, P. R. Human Appropriation of Renewable Fresh Water. Science. 271, 785–788 (1996).
United Nations Children’s Fund (UNICEF) & World Health Organization (WHO). Progress on household drinking water, sanitation and hygiene 2000-2017. Special focus on inequalities. https://www.unicef.org/media/55276/file/Progress on drinking water, sanitation and hygiene 2019.pdf (2019).
Prüss-Ustün, A. et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health 222, 765 (2019).
Caprioli, A., Morabito, S., Bruégre, H. & Oswald, E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36, 289–311 (2005).
Vital, M., Fuchslin, H. P., Hammes, F. & Egli, T. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 153, 1993–2001 (2007).
Agudelo Higuita, N. I. & Huycke, M. M. Enterococcal Disease, Epidemiology, and Implications for Treatment. in Enterococci: From Commensals to Leading Causes of Drug Resistant Infection 47–72 (Massachusetts Eye and Ear Infirmary, 2014).
Paton, J. C. & Paton, A. W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11, 450–479 (1998).
Bellamy, W. D., Silverman, G. P., Hendricks, D. W. & Logsdon, G. S. Removing Giardia cysts with slow sand filtration. J. Am. Water Works Assoc. 77, 52–60 (1985).
Fogel, D., Isaac-Renton, J., Guasparini, R., Moorehead, W. & Removing, O. J. giardia and cryptosporidium by slow sand filtration. JAWWA, Res. Technol. 3, 77–84 (1993).
Hijnen, W. A. M., Schijven, J. F., Bonné, P., Visser, A. & Medema, G. J. Elimination of viruses, bacteria and protozoan oocysts by slow sand filtration. Water Sci. Technol. 50, 147–154 (2004).
Campos, L. C., Su, M. F. J., Graham, N. J. D. & Smith, S. R. Biomass development in slow sand filters. Water Res. 36, 4543–4551 (2002).
Basu, O. D., Dhawan, S. & Black, K. Applications of biofiltration in drinking water treatment - a review. J. Chem. Technol. Biotechnol. 91, 585–595 (2016).
Terry, L. G. & Summers, R. S. Biodegradable organic matter and rapid-rate biofilter performance: A review. Water Res. 128, 234–245 (2018).
Loh, Z. Z. et al. Shifting from conventional to organic filter media in wastewater biofiltration treatment: a review. Appl. Sci. 2021, Vol. 11, Page 8650 11, 8650 (2021).
Bennett, A. Drinking water: Pathogen removal from water – technologies and techniques. Filtr. Sep. 45, 14–16 (2008).
Di Cristo, C., Esposito, G. & Leopardi, A. Modelling trihalomethanes formation in water supply systems. Environ. Technol. 34, 61–70 (2013).
Pooi, C. K. & Ng, H. Y. Review of low-cost point-of-use water treatment systems for developing communities. npj Clean Water 2018 11 1, 11 (2018).
Flemming, H.-C. et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
Fu, J. et al. Pilot investigation of two-stage biofiltration for removal of natural organic matter in drinking water treatment. Chemosphere 166, 311–322 (2017).
Chen, F. et al. Kinetics of natural organic matter (NOM) removal during drinking water biofiltration using different NOM characterization approaches. Water Res. 104, 361–370 (2016).
McKie, M. J., Ziv-El, M. C., Taylor-Edmonds, L., Andrews, R. C. & Kirisits, M. J. Biofilter scaling procedures for organics removal: A potential alternative to piloting. Water Res. 151, 87–97 (2019).
de Vries, J. Soil filtration of wastewater effluent and the mechanism of pore clogging. J. Water Pollut. Control Fed. 44, 565–573 (1972).
Métivier, R., Bourven, I., Labanowski, J. & Guibaud, G. Interaction of erythromycin ethylsuccinate and acetaminophen with protein fraction of extracellular polymeric substances (EPS) from various bacterial aggregates. Environ. Sci. Pollut. Res. 20, 7275–7285 (2013).
Writer, J. H., Barber, L. B., Ryan, J. N. & Bradley, P. M. Biodegradation and attenuation of steroidal hormones and alkylphenols by stream biofilms and sediments. Environ. Sci. Technol. 45, 4370–4376 (2011).
Flemming, H.-C. Biofilms. in Encyclopedia of Life Sciences (John Wiley & Sons, Ltd, 2008). https://doi.org/10.1002/9780470015902.a0000342.pub2.
Kragh, K. N. et al. Role of multicellular aggregates in biofilm formation. MBio 7, e00237 (2016).
Grumbein, S., Opitz, M. & Lieleg, O. Selected metal ions protect Bacillus subtilis biofilms from erosion †. Metallomics 6, 1441 (2014).
Fu, J. et al. Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment. Sci. Total Environ. 664, 240–248 (2019).
Nemani, V. A., McKie, M. J., Taylor-Edmonds, L. & Andrews, R. C. Impact of biofilter operation on microbial community structure and performance. J. Water Process Eng. 24, 35–41 (2018).
Beutel, M. W. & Larson, L. Pathogen removal from urban pond outflow using rock biofilters. Ecol. Eng. 78, 72–78 (2014).
Wendt, C. et al. Microbial removals by a novel biofilter water treatment system. Am. J. Trop. Med. Hyg. 92, 765–772 (2015).
Granger, H. C., Stoddart, A. K. & Gagnon, G. A. Direct biofiltration for manganese removal from surface water. J. Environ. Eng. 140, 04014006 (2014).
Srivastava, N. K. & Majumder, C. B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 151, 1–8 (2008).
Fu, J. et al. Removal of disinfection byproduct (DBP) precursors in water by two-stage biofiltration treatment. Water Res. 123, 224–235 (2017).
McKie, M. J., Andrews, S. A. & Andrews, R. C. Conventional drinking water treatment and direct biofiltration for the removal of pharmaceuticals and artificial sweeteners: A pilot-scale approach. Sci. Total Environ. 544, 10–17 (2016).
Crognale, S. et al. Biological As(III) oxidation in biofilters by using native groundwater microorganisms. Sci. Total Environ. 651, 93–102 (2019).
Klayman, B. J., Volden, P. A., Stewart, P. S. & Camper, A. K. Escherichia coli O157:H7 requires colonizing partner to adhere and persist in a capillary flow cell. Environ. Sci. Technol. 43, 2105–2111 (2009).
Bauman, W. J., Nocker, A., Jones, W. L. & Camper, A. K. Retention of a model pathogen in a porous media biofilm. Biofouling 25, 229–240 (2009).
Nocker, A., Burr, M. & Camper, A. Pathogens in water and biofilms. In Microbiology of waterborne diseases: microbiological aspects and risks: Second Edition 3–32 (Academic Press, 2013). https://doi.org/10.1016/B978-0-12-415846-7.00001-9.
Li, J., McLellan, S. & Ogawa, S. Accumulation and fate of green fluorescent labeled Escherichia coli in laboratory-scale drinking water biofilters. Water Res. 40, 3023–3028 (2006).
Rendueles, O. & Ghigo, J.-M. Mechanisms of competition in biofilm communities. Microbiol. Spectr. 3, 1–14 (2015).
Hibbing, M. E., Fuqua, C., Parsek, M. R. & Peterson, S. B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25 (2010).
Aoki, S. K. et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468, 439–442 (2010).
MacIntyre, D. L., Miyata, S. T., Kitaoka, M. & Pukatzki, S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl Acad. Sci. U.S.A. 107, 19520–19524 (2010).
Ławniczak, Ł., Marecik, R. & Chrzanowski, Ł. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 97, 2327 (2013).
Cornforth, D. M. & Foster, K. R. Competition sensing: the social side of bacterial stress responses. Nat. Rev. Microbiol. 2013 114 11, 285–293 (2013).
Legnani, P., Leoni, E., Rapuano, S., Turin, D. & Valenti, C. Survival and growth of Pseudomonas aeruginosa in natural mineral water: a 5-year study. Int. J. Food Microbiol. 53, 153–158 (1999).
Moll, D. M., Summers, R. S., Fonseca, A. C. & Matheis, W. Impact of temperature on drinking water biofilter performance and microbial community structure. Environ. Sci. Technol. 33, 2377–2382 (1999).
Hozalski, R. M., Bouwer, E. J. & Goel, S. Removal of natural organic matter (NOM) from drinking water supplies by ozone-biofiltration. Water Sci. Technol. 40, 157–163 (1999).
Schmidt, K. D., Tümmler, B. & Römling, U. Comparative genome mapping of Pseudomonas aeruginosa PAO with P. aeruginosa C, which belongs to a major clone in cystic fibrosis patients and aquatic habitats. J. Bacteriol. 178, 85 (1996).
Nigaud, Y. et al. Biofilm-induced modifications in the proteome of Pseudomonas aeruginosa planktonic cells. Biochim. Biophys. Acta - Proteins Proteom. 1804, 957–966 (2010).
Von Ohle, C. et al. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl. Environ. Microbiol. 76, 2326 (2010).
Nescerecka, A., Juhna, T. & Hammes, F. Identifying the underlying causes of biological instability in a full-scale drinking water supply system. Water Res. 135, 11–21 (2018).
Prest, E. I., Hammes, F., Kötzsch, S., Van Loosdrecht, M. C. M. & Vrouwenvelder, J. S. A systematic approach for the assessment of bacterial growth-controlling factors linked to biological stability of drinking water in distribution systems. Water Sci. Technol. Water Supply 16, 865–880 (2016).
Liang, K., Sobsey, M. & Stauber, C. E. Improving Household Drinking Water Quality: Use of Biosand Filter in Cambodia. https://scholarworks.gsu.edu/iph_facpub (2010).
Fabiszewski De Aceituno, A. M., Stauber, C. E., Walters, A. R., Meza Sanchez, R. E. & Sobsey, M. D. A randomized controlled trial of the plastic-housing biosand filter and its impact on diarrheal disease in Copan, Honduras. Am. J. Trop. Med. Hyg. 86, 913–921 (2012).
Miettinen, I. T., Vartiainen, T. & Martikainen, P. J. Phosphorus and bacterial growth in drinking water. Appl. Environ. Microbiol. 63, 3242–3245 (1997).
Keinänen, M. M. et al. The microbial community structure of drinking water biofilms can be affected by phosphorus availability. Appl. Environ. Microbiol. 68, 434–439 (2002).
United Nations (UN). Transforming Our World: The 2030 Agenda for Sustainable Development. in A New Era in Global Health 529–567, https://doi.org/10.1891/9780826190123.ap02 (2018).
Serra, M. O. D. E. & Schnitzer, M. Extraction of humic acid by alkali and chelating resin. Can. J. Soil Sci. 52, 365–374 (1972).
Smith, E. J., Davison, W. & Hamilton-Taylor, J. Methods for preparing synthetic freshwaters. Water Res. 36, 1286–1296 (2002).
Sobsey, M. D. Managing Water in the Home: Accelerated Health Gains from Improved Water Supply Water, Sanitation and Health Department of Protection of the Human Environment World Health Organization Geneva. https://apps.who.int/iris/bitstream/handle/10665/67319/WHO_SDE_WSH_02.07.pdf?sequence=1&isAllowed=y (2002).
Carratalà, A. et al. Solar disinfection of viruses in polyethylene terephthalate bottles. Appl. Environ. Microbiol. 82, 279–288 (2016).
Attisani, M. Can solar technology generate clean water for developing nations? Renew. Energy Focus 17, 138–139 (2016).
Chaidez, C. et al. Point-of-use Unit Based on Gravity Ultrafiltration Removes Waterborne Gastrointestinal Pathogens from Untreated Water Sources in Rural Communities. Wilderness Environ. Med. 27, 379–385 (2016).
Clayton, G. E., Thorn, R. M. S. & Reynolds, D. M. Development of a novel off-grid drinking water production system integrating electrochemically activated solutions and ultrafiltration membranes. J. Water Process Eng. 30, 100480 (2017).
Baig, S. A., Mahmood, Q., Nawab, B., Shafqat, M. N. & Pervez, A. Improvement of drinking water quality by using plant biomass through household biosand filter - A decentralized approach. Ecol. Eng. 37, 1842–1848 (2011).
Acknowledgements
Andrew Cox for providing technical advice. This research was funded by UWE, Bristol, and Origin Aqua Technologies as part of a partnership Ph.D. and partially supported by the Natural Environment Research Council, UK [NE/R003106/1].
Author information
Authors and Affiliations
Contributions
J.A.C.S.: experimental method development, experimental work, data collection, and analysis, manuscript preparation and writing, review and editing. R.M.S.: funding acquisition, experimental conceptualisation and development, data analysis, project supervision and manuscript review and editing. G.M.R.: project supervision, data analysis and manuscript review and editing. D.T.: project supervision, data analysis, and manuscript review and editing. J.E.L.: experimental conceptualisation, manuscript review and editing. D.M.R.: funding acquisition, experimental conceptualisation and development, data analysis, project supervision and manuscript review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steven, J.A.C., Thorn, R.M.S., Robinson, G.M. et al. The control of waterborne pathogenic bacteria in fresh water using a biologically active filter. npj Clean Water 5, 30 (2022). https://doi.org/10.1038/s41545-022-00169-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-022-00169-y