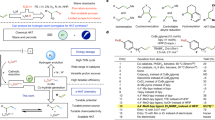
Abstract
Metal-free catalysts offer a desirable alternative to traditional metal-based electrocatalysts. However, metal-free catalysts, featuring defined active sites, rarely show activities as promising as metal-based materials. Here we report 2-thiolimidazole as an efficient metal-free catalyst for selective electrocatalytic hydrogenation of acetylene into ethylene. Under alkaline conditions, the sulfhydryl and imino groups of 2-thiolimidazole are spontaneously deprotonated into dianions. Deprotonation thus enriches the negative charges of pyridinic N sites in 2-thiolimidazole to enhance the adsorption of electrophilic acetylene through the σ-configuration. Ethylene partial current densities show a volcano relationship with the negative charges of the pyridinic N sites in various imidazole derivatives. Consequently, the deprotonated 2-thiolimidazole exhibits an ethylene partial current density and faradaic efficiency competitive with metal-based catalysts like Cu and Pd. This work highlights the tunability and promising potential of metal-free molecules in electrocatalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329–338 (2019).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Gong, M. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 5, 4695 (2014).
Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5, 300–306 (2013).
Duchesne, P. N. et al. Golden single-atomic-site platinum electrocatalysts. Nat. Mater. 17, 1033–1039 (2018).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Gong, K., Du, F., Xia, Z., Durstock, M. & Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323, 760–764 (2009).
Jiao, Y., Zheng, Y., Davey, K. & Qiao, S.-Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 1, 16130 (2016).
Kumar, B. et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 4, 2819 (2013).
Zhang, J., Zhao, Z., Xia, Z. & Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015).
Zhang, S. et al. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J. Am. Chem. Soc. 136, 7845–7848 (2014).
Guo, D. et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351, 361–365 (2016).
Zhao, Y. et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis. Nat. Chem. 10, 924–931 (2018).
Cui, P., Zhao, L., Long, Y., Dai, L. & Hu, C. Carbon-based electrocatalysts for acidic oxygen reduction reaction. Angew. Chem. Int. Ed. 62, e202218269 (2023).
Xue, L. et al. Zigzag carbon as efficient and stable oxygen reduction electrocatalyst for proton exchange membrane fuel cells. Nat. Commun. 9, 3819 (2018).
Shui, J., Wang, M., Du, F. & Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 1, e1400129 (2015).
Zheng, Y. et al. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 5, 3783 (2014).
Wu, J. et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 7, 13869 (2016).
Xie, J. et al. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution. Angew. Chem. Int. Ed. 57, 9640–9644 (2018).
Pan, B. et al. Toward highly selective electrochemical CO2 reduction using metal-free heteroatom-doped carbon. Adv. Sci. 7, 2001002 (2020).
Huang, L. et al. Direct synthesis of ammonia from nitrate on amorphous graphene with near 100% efficiency. Adv. Mater. 35, e2211856 (2023).
Zhang, C. et al. A pentagonal defect-rich metal-free carbon electrocatalyst for boosting acidic O2 reduction to H2O2 production. J. Am. Chem. Soc. 145, 11589–11598 (2023).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Yang, J. et al. CO2-mediated organocatalytic chlorine evolution under industrial conditions. Nature 617, 519–523 (2023).
Wu, S. et al. Highly durable organic electrode for sodium-ion batteries via a stabilized α-C radical intermediate. Nat. Commun. 7, 13318 (2016).
Janoschka, T. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 527, 78–81 (2015).
Wang, S. et al. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. Nat. Commun. 12, 7072 (2021).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4, 565–574 (2021).
Zhao, B.-H. et al. Economically viable electrocatalytic ethylene production with high yield and selectivity. Nat. Sustain. 6, 827–837 (2023).
Bu, J. et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. Nat. Catal. 4, 557–564 (2021).
Zhang, L. et al. Efficient electrocatalytic acetylene semihydrogenation by electron-rich metal sites in N-heterocyclic carbene metal complexes. Nat. Commun. 12, 6574 (2021).
Xue, G., Huang, X.-Y., Dong, J. & Zhang, J. The formation of an effective anti-corrosion film on copper surfaces from 2-mercaptobenzimidazole solution. J. Electroanal. Chem. 310, 139–148 (1991).
Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 72, 90–98 (2013).
Platzer, G., Okon, M. & McIntosh, L. P. pH-dependent random coil 1H, 13C, and 15N chemical shifts of the ionizable amino acids: a guide for protein pKa measurements. J. Biomol. NMR 60, 109–129 (2014).
Richmond, W. N., Faguy, P. W. & Weibel, S. C. An in situ infrared spectroscopic study of imidazole films on copper electrodes. J. Electroanal. Chem. 448, 237–244 (1998).
Biswas, N., Thomas, S., Sarkar, A., Mukherjee, T. & Kapoor, S. Adsorption of methimazole on silver nanoparticles: FTIR, Raman, and surface-enhanced Raman scattering study aided by density functional theory. J. Phys. Chem. C 113, 7091–7100 (2009).
Chandra, S., Chowdhury, J., Ghosh, M. & Talapatra, G. B. Genesis of enhanced Raman bands in SERS spectra of 2-mercaptoimidazole: FTIR, Raman, DFT, and SERS. J. Phys. Chem. A 116, 10934–10947 (2012).
Wang, X., Xu, C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019).
Mahmood, N. et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. 5, 1700464 (2018).
Guan, Q. et al. Reactive metal–biopolymer interactions for semihydrogenation of acetylene. ACS Catal. 9, 11146–11152 (2019).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Andrae, D., Häußermann, U., Dolg, M., Stoll, H. & Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theoret. Chim. Acta 77, 123–141 (1990).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell Cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Weinhold, F. & Landis C. R. Discovering Chemistry with Natural Bond Orbitals (John Wiley & Sons, 2012).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52373308 (J.Z.), 22005245 (J.Z.), 52101271 (L.Z.) and 22202123 (Z.W.)), the Key Research and Development Program of Shaanxi Province (2023-YBGY-284 (J.Z.) and 2024GX-YBXM-379 (L.Z.)), the Fundamental Research Funds for the Central Universities (G2022KY0606 (J.Z.) and G2020KY05306 (L.Z.)) and the Guangdong Basic and Applied Basic Research Foundation (2020A1515111017 (L.Z.)). We thank C. Liu at Shanxi University for help with the simulations.
Author information
Authors and Affiliations
Contributions
L.Z. and J.Z. conceived and designed the experiments. J.Z. supervised the project. L.Z. and R.B. carried out experiments and analysed the results. J.L. and Z.L. performed the characterization of the catalyst. J.B. optimized the electrochemical and product analysis set-ups. S.A. contributed to the electrochemical measurements. Z.W. conducted the grand canonical calculations. L.Z. and J.Z. assembled the figures and cowrote the manuscript. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Liang Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1–5 and Discussion.
Supplementary Data 1
Statistical source data for Supplementary Fig. 1.
Supplementary Data 2
Statistical source data for Supplementary Fig. 3.
Supplementary Data 3
Statistical source data for Supplementary Fig. 4.
Supplementary Data 4
Statistical source data for Supplementary Fig. 5.
Supplementary Data 5
Statistical source data for Supplementary Fig. 7.
Supplementary Data 6
Statistical source data for Supplementary Fig. 9.
Supplementary Data 7
Statistical source data for Supplementary Fig. 12.
Supplementary Data 8
Statistical source data for Supplementary Fig. 16.
Supplementary Data 9
Statistical source data for Supplementary Fig. 26.
Supplementary Data 10
Statistical source data for Supplementary Fig. 27.
Supplementary Data 11
Statistical source data for Supplementary Fig. 31.
Supplementary Data 12
Statistical source data for Supplementary Fig. 36.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Bai, R., Lin, J. et al. Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01480-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01480-6