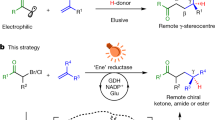
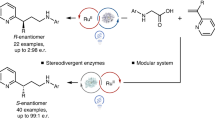
Abstract
Photobiocatalysis—where light is used to expand the reactivity of an enzyme—has recently emerged as a powerful strategy to develop chemistries that are new to nature. These systems have shown potential in asymmetric radical reactions that have long eluded small-molecule catalysts1. So far, unnatural photobiocatalytic reactions are limited to overall reductive and redox-neutral processes2,3,4,5,6,7,8,9. Here we report photobiocatalytic asymmetric sp3–sp3 oxidative cross-coupling between organoboron reagents and amino acids. This reaction requires the cooperative use of engineered pyridoxal biocatalysts, photoredox catalysts and an oxidizing agent. We repurpose a family of pyridoxal-5′-phosphate-dependent enzymes, threonine aldolases10,11,12, for the α-C–H functionalization of glycine and α-branched amino acid substrates by a radical mechanism, giving rise to a range of α-tri- and tetrasubstituted non-canonical amino acids 13,14,15 possessing up to two contiguous stereocentres. Directed evolution of pyridoxal radical enzymes allowed primary and secondary radical precursors, including benzyl, allyl and alkylboron reagents, to be coupled in an enantio- and diastereocontrolled fashion. Cooperative photoredox–pyridoxal biocatalysis provides a platform for sp3–sp3 oxidative coupling16, permitting the stereoselective, intermolecular free-radical transformations that are unknown to chemistry or biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text and the Supplementary Information. Plasmids encoding PLP radical enzymes reported in this study are available for research purposes from Y.Y. under a material transfer agreement with the University of California Santa Barbara.
Change history
17 May 2024
In the version of the article initially published, the Supplementary Data files (NMR spectra and X-ray data) were missing and are now available in the HTML version of the article.
References
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166 (2019).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Ye, Y. et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination. Nat. Chem. 15, 206–212 (2023).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Liu, J.-Q. et al. Diversity of microbial threonine aldolases and their application. J. Mol. Catal. B: Enzym. 10, 107–115 (2000).
Dückers, N., Baer, K., Simon, S., Gröger, H. & Hummel, W. Threonine aldolases—screening, properties and applications in the synthesis of non-proteinogenic β-hydroxy-α-amino acids. Appl. Microbiol. Biotechnol. 88, 409–424 (2010).
Fesko, K., Strohmeier, G. A. & Breinbauer, R. Expanding the threonine aldolase toolbox for the asymmetric synthesis of tertiary α-amino acids. Appl. Microbiol. Biotechnol. 99, 9651–9661 (2015).
Nájera, C. & Sansano, J. M. Catalytic asymmetric synthesis of α-amino acids. Chem. Rev. 107, 4584–4671 (2007).
Almhjell, P. J., Boville, C. E. & Arnold, F. H. Engineering enzymes for noncanonical amino acid synthesis. Chem. Soc. Rev. 47, 8980–8997 (2018).
Hickey, J. L., Sindhikara, D., Zultanski, S. L. & Schultz, D. M. Beyond 20 in the 21st century: prospects and challenges of non-canonical amino acids in peptide drug discovery. ACS Med. Chem. Lett. 14, 557–565 (2023).
Lei, A. et al. Oxidative Cross-Coupling Reactions 1st edn (Wiley, 2016).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Fu, Y. et al. Engineered P450 atom-transfer radical cyclases are bifunctional biocatalysts: reaction mechanism and origin of enantioselectivity. J. Am. Chem. Soc. 144, 13344–13355 (2022).
Fu, W. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat. Catal. 6, 628–636 (2023).
Eliot, A. C. & Kirsch, J. F. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu. Rev. Biochem. 73, 383–415 (2004).
Du, Y.-L. & Ryan, K. S. Pyridoxal phosphate-dependent reactions in the biosynthesis of natural products. Nat. Prod. Rep. 36, 430–457 (2019).
Hedges, J. B. & Ryan, K. S. Biosynthetic pathways to nonproteinogenic α-amino acids. Chem. Rev. 120, 3161–3209 (2020).
Dumas, A., Lercher, L., Spicer, C. D. & Davis, B. G. Designing logical codon reassignment – expanding the chemistry in biology. Chem. Sci. 6, 50–69 (2015).
Wang, Q., Gu, Q. & You, S.-L. Enantioselective carbonyl catalysis enabled by chiral aldehydes. Angew. Chem. Int. Ed. 58, 6818–6825 (2019).
Maier, T. H. P. Semisynthetic production of unnatural l-α-amino acids by metabolic engineering of the cysteine-biosynthetic pathway. Nat. Biotechnol. 21, 422–427 (2003).
Phillips, R. S. Synthetic applications of tryptophan synthase. Tetrahedron: Asymmetry 15, 2787–2792 (2004).
Hai, Y., Chen, M., Huang, A. & Tang, Y. Biosynthesis of mycotoxin fusaric acid and application of a PLP-dependent enzyme for chemoenzymatic synthesis of substituted l-pipecolic acids. J. Am. Chem. Soc. 142, 19668–19677 (2020).
Frey, P. A. & Reed, G. H. Pyridoxal-5′-phosphate as the catalyst for radical isomerization in reactions of PLP-dependent aminomutases. Biochim. Biophys. Acta 1814, 1548–1557 (2011).
Hoffarth, E. R., Rothchild, K. W. & Ryan, K. S. Emergence of oxygen- and pyridoxal phosphate-dependent reactions. FEBS J. 287, 1403–1428 (2020).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Contestabile, R. et al. l-threonine aldolase, serine hydroxymethyltransferase and fungal alanine racemase. Eur. J. Biochem. 268, 6508–6525 (2001).
Schaffer, J. E., Reck, M. R., Prasad, N. K. & Wencewicz, T. A. β-Lactone formation during product release from a nonribosomal peptide synthetase. Nat. Chem. Biol. 13, 737–744 (2017).
Scott, T. A., Heine, D., Qin, Z. & Wilkinson, B. An l-threonine transaldolase is required for l-threo-β-hydroxy-α-amino acid assembly during obafluorin biosynthesis. Nat. Comm. 8, 15935 (2017).
Xu, L., Wang, L.-C., Xu, X.-Q. & Lin, J. Characteristics of l-threonine transaldolase for asymmetric synthesis of β-hydroxy-α-amino acids. Catal. Sci. Technol. 9, 5943–5952 (2019).
Kumar, P. et al. l-threonine transaldolase activity is enabled by a persistent catalytic intermediate. ACS Chem. Biol. 16, 86–95 (2021).
Gao, J. et al. A pyridoxal 5′-phosphate-dependent Mannich cyclase. Nat. Catal. 6, 476–486 (2023).
Flamigni, L., Barbieri, A., Sabatini, C., Ventura, B. & Barigelletti, F. in Photochemistry and Photophysics of Coordination Compounds II (eds Balzani, V. & Campagna, S.) 143–203 (Springer, 2007).
Bard, A. J. et al. (eds) Standard Potentials in Aqueous Aolution 1st edn (Routledge, 1985).
Liu, J.-Q. et al. Gene cloning, biochemical characterization and physiological role of a thermostable low-specificity l-threonine aldolase from Escherichia coli. Eur. J. Biochem. 255, 220–226 (1998).
Liu, J. Q., Dairi, T., Kataoka, M., Shimizu, S. & Yamada, H. l-allo-threonine aldolase from Aeromonas jandaei DK-39: gene cloning, nucleotide sequencing, and identification of the pyridoxal 5’-phosphate-binding lysine residue by site-directed mutagenesis. J. Bacteriol. 179, 3555–3560 (1997).
Fesko, K., Uhl, M., Steinreiber, J., Gruber, K. & Griengl, H. Biocatalytic access to α,α-dialkyl-α-amino acids by a mechanism-based approach. Angew. Chem. Int. Ed. 49, 121–124 (2010).
Kielkopf, C. L. & Burley, S. K. X-ray structures of threonine aldolase complexes: structural basis of substrate recognition. Biochemistry 41, 11711–11720 (2002).
Li, F., Yang, L.-C., Zhang, J., Chen, J. S. & Renata, H. Stereoselective synthesis of β-branched aromatic α-amino acids by biocatalytic dynamic kinetic resolution. Angew. Chem. Int. Ed. 60, 17680–17685 (2021).
Guo, F. & Berglund, P. Transaminase biocatalysis: optimization and application. Green Chem. 19, 333–360 (2017).
Parmeggiani, F., Weise, N. J., Ahmed, S. T. & Turner, N. J. Synthetic and therapeutic applications of ammonia-lyases and aminomutases. Chem. Rev. 118, 73–118 (2018).
Lennox, A. J. J., Nutting, J. E. & Stahl, S. S. Selective electrochemical generation of benzylic radicals enabled by ferrocene-based electron-transfer mediators. Chem. Sci. 9, 356–361 (2018).
Tantillo, D. J., Chen, J. & Houk, K. N. Theozymes and compuzymes: theoretical models for biological catalysis. Curr. Opin. Chem. Biol. 2, 743–750 (1998).
Acknowledgements
This research is supported by the NIH (R35GM147387 to Y.Y. and R35GM128779 to P.L.) and the Packard Foundation (Y.Y.). PLP enzyme mining is supported by the Herman Frasch Foundation (947-HF22 to Y.Y.). We acknowledge the NSF BioPACIFIC MIP (DMR-1933487) and NSF MRSEC at UCSB (DMR-2308708) for access to instrumentation. Computational studies were carried out at the University of Pittsburgh Center for Research Computing and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) programme, supported by NSF award numbers OAC-2117681, OAC-1928147 and OAC-1928224. We are grateful to Y. Wang (University of Pittsburgh) for critical reading of this manuscript as well as P. C. Ford (University of California Santa Barbara) and Q. Zhu (University of Utah) for helpful discussions on photo- and electrochemistry.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and directed the project. T.-C.W. discovered and optimized the oxidative photobiocatalytic process. T.-C.W. and Z.Z. performed the directed evolution and carried out the substrate scope studies. Z.B. and J.L. prepared the organoboron substrates. B.K.M. carried out the computational studies, with P.L. and Y.Y. providing guidance. Y.Y., P.L. and B.K.M. wrote the manuscript with the input of all other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.Y., T.-C.W. and Z.Z. are inventors on a patent application submitted by the University of California Santa Barbara that covers compositions, methods and applications of biocatalytic non-canonical amino acid synthesis. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Mechanistic studies.
a, TEMPO trapping studies. Reaction conditions: 1a’ (1.0 equiv, 3.0 mM), 2a (10 equiv, 30.0 mM), 1 mol% TmPLPα1 (30 μM), 10 mol% PLP (300 μM), 2 mol% (fac)-Ir(ppy)3 (60 μM), Co(NH3)6Cl3 (2.0 equiv, 6.0 mM), TEMPO (3.0 equiv, 9.0 mM), hν (440 nm), 200 mM KPi buffer, 50 °C, 12 h. b, Radical generation studies. Reaction conditions: 1a’ (1.0 equiv, 3.0 mM), 2 mol% (fac)-Ir(ppy)3 (60 μM), Co(NH3)6Cl3 (2.0 equiv, 6.0 mM), hν (440 nm), 200 mM KPi buffer, 50 °C, 12 h.
Extended Data Fig. 2 Computational studies on threonine aldolase-catalysed oxidative cross-coupling.
a, Computed energy profile using a theozyme model at the (U)ωB97X-D/6-311+G(2d,2p)-SDD(Ir)/SMD(PhCl)//(U)B3LYP-D3/6-31G(d)-SDD(Ir) level of theory. Except those in external aldimine 13, active-site residues are omitted for clarity. Enthalpy values (ΔH) are with respect to 13. b, Activation enthalpies for radical additions to quinonoid species computed using theozyme and a cofactor-only model. Enthalpies are relative to the van der Waals complex (15). c, Optimized structures of regio- and enantioselectivity-determining radical addition transition states.
Extended Data Fig. 3 UV-vis spectroscopic analysis of threonine aldolase variants upon the introduction of D-alanine, L-alanine and glycine.
a, TmTA W86N (TmPLPα1) at pH 8. b, TmTA W86N (TmPLPα1) at pH 9.
Supplementary information
Supplementary Information
Experimental procedures, methods, supplementary figures and tables, and characterization data.
Supplementary Data 1
NMR spectra.
Supplementary Data 2
X-ray data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, TC., Mai, B.K., Zhang, Z. et al. Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling. Nature 629, 98–104 (2024). https://doi.org/10.1038/s41586-024-07284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07284-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.