Abstract
Mapping the localization of the functional brain regions in trigeminal neuralgia (TN) patients is still lacking. The study aimed to explore the functional brain alterations and influencing factors in TN patients using functional brain imaging techniques. All participants underwent functional brain imaging to collect resting-state brain activity. The significant differences in regional homogeneity (ReHo) and amplitude of low frequency (ALFF) between the TN and control groups were calculated. After familywise error (FWE) correction, the differential brain regions in ReHo values between the two groups were mainly located in bilateral middle frontal gyrus, bilateral inferior cerebellum, right superior orbital frontal gyrus, right postcentral gyrus, left inferior temporal gyrus, left middle temporal gyrus, and left gyrus rectus. The differential brain regions in ALFF values between the two groups were mainly located in the left triangular inferior frontal gyrus, left supplementary motor area, right supramarginal gyrus, and right middle frontal gyrus. With the functional impairment of the central pain area, the active areas controlling memory and emotion also change during the progression of TN. There may be different central mechanisms in TN patients of different sexes, affected sides, and degrees of nerve damage. The exact central mechanisms remain to be elucidated.
Similar content being viewed by others
Introduction
Trigeminal neuralgia (TN) is a chronic brain disease involving the trigeminal nerve1. Neurovascular compression (NVC) in the root entry zone of the brainstem and the excitability of the nerve fibers involved are the potential causes2,3. However, NVC of the trigeminal nerve is present on both the asymptomatic and symptomatic sides in most TN patients4. Many TN patients with substantial NVC fail to achieve long-term pain relief after technically successful surgery5,6. In this way, the peripheral mechanisms alone do not adequately explain the onset of TN. It is, therefore, essential to explore the central mechanisms of TN. Mapping the localization of the functional brain regions in TN patients is still lacking.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a suitable technique for studying central mechanisms. It enables direct observation of subjects’ spontaneous neurological brain activity at rest without other activity, emphasizing the parallelism of the human neural network and the significance of the relationships between brain regions7. The amplitude of low frequency (ALFF) is a valuable metric based on rs-fMRI that can be used to assess the intrinsic fluctuations of the signal to reflect the intensity of spontaneous neural activity8. Changes in ALFF values are often thought to be related to metabolic levels9. Regional homogeneity (ReHo) was applied to investigate the coherence of spontaneous neuronal activity between a given voxel and its neighbors at rest, further clarifying differences in local brain activity. Changes in ReHo values reveal possible abnormalities in the synchronization and coordination of spontaneous neuronal activity. Thus, ALFF and ReHo have become practical tools for measuring brain structure and better understanding pathophysiological changes in brain function.
The default mode network (DMN) is the most stable and well-studied resting-state brain network. It is generally divided into three subsystems: medial prefrontal region, lateral parietal cortex and precuneus regions, and medial parietal and posterior cingulate regions. In addition to functions such as memory and cognition, the DMN is involved in various cognitive tasks, including those unrelated to task stimuli9,10,11. It is usually active when not in contact with the external environment12. The DMN is involved in mediating pain feedback. In turn, Potential alterations in DMN activity may be related to symptoms (in addition to pain) often exhibited by people with chronic pain, including anxiety, depression, sleep disturbances, and abnormal decision-making13, which greatly reduce their quality of life. Neuropathic pain is a common form of chronic pain caused by lesions or diseases of the somatosensory nervous system14. Accumulating evidence suggests that the brain’s DMN and somatosensory systems play a vital role in the pathophysiology of chronic pain in humans9,15,16,17,18,19,20. The study aimed to explore the functional brain alterations and influencing factors in TN patients using functional brain imaging techniques.
Materials and methods
Ethics statement
The study was approved (2022-R281) by the Ethics Committee of the Second Hospital of Hebei Medical University, Hebei, China, and subjects signed informed consent to participate in the study. The study was carried out in accordance with the Declaration of Helsinki.
Participants
Thirty-eight TN patients (TN group; 11 males and 27 females; age 62.2 ± 10.3 years) and 20 healthy controls (control group; 8 males and 13 females; age 56.8 ± 9.4 years) were included in this study. Inclusion criteria were (i) unilateral pain involving one or more branches of the trigeminal nerve; (ii) paroxysmal facial pain lasting from seconds to minutes; (iii) sharp, burning or piercing pain with characteristic trigger points or trigger factors; (iv) routine magnetic resonance imaging (MRI) examination showing no obvious abnormal brain signal; (v) no obvious clinical neurological or sensory dysfunction; (vi) duration of more than six months (chronic pain patients) and (vii) right-handed. The diagnosis of TN was determined according to the International Classification of Headache Disorders (3rd Edition)1. The exclusion criteria were (i) migraine or other paroxysmal or chronic pain; (ii) secondary TN caused by intracranial tumors, multiple sclerosis or other diseases; (iii) neurological or psychiatric diseases, including traumatic brain injury, brain tumors, cerebral hemorrhage and other neurological and psychiatric conditions; (iv) history of alcohol or drug abuse; and (v) contraindications to magnetic resonance scanning.
Pain evaluation
An evaluation of TN pain was conducted prior to MRI scan using the visual analog scale (VAS). By using a 10 cm ruler, patients rated their pain from 0 to 10. An evaluation of 0 means no pain, and a rating of 10 means severe, extremely intolerable pain21.
MRI data collection
For the MRI scan, a Philips Achieva 3.0 T X-series MRI machine was used for scanning with an eight-channel phase array head coil. Participants were instructed to close their eyes, remain awake, and breathe quietly until the scan was complete. The scanning sequence included three-dimensional fast imaging (3D-DRIVE), high resolution (HR) 3D T1 weighted imaging (T1WI), and rs-fMRI. The parameters of the 3D-DRIVE were set as follows: repetition time (TR)/echo time (TE) 1500/200 ms; field of view (FOV) 200 mm × 200 mm; slice thickness 0.5 mm; slice spacing 0 mm; matrix 640 × 640. The scanning baseline was parallel to the auditory-orbital line and extended from the frontal base to the bridging sulcus, including the trigeminal nerve, facial auditory nerve, and the main branches of the vertebrobasilar artery. The 3D-DRIVE sequence was reconstructed in the oblique sagittal and coronal positions. HR structural images were collected using a sagittal fast field echo (FFE) sequence with the following parameters: TR/TE 7.61/3.71 ms, number of excitations (NEX) 1, flip angle (FA) 8°, FOV 240 mm × 240 mm, matrix 240 × 240, voxel 1 mm × 1 mm, slice thickness 1 mm, and slice spacing 0 mm. Gradient-recalled echo (GRE) echo-planar imaging (EPI) sequences were used to collect functional data. The parameters were as follows: TR/TE 2000/30 ms, NEX 1, FA 90°, FOV 240 mm × 240 mm, acquisition matrix 64 × 64, voxel 3.75 mm × 3.75 mm × 4.0 mm, number of plies 35, slice thickness 4.0 mm, slice spacing 0 mm, Each scanning process lasted 8 min; and 240 time points were collected. Under the premise of constant acquisition time, parallel acquisition technology is adopted to reduce magnetic sensitive artifacts.
Evaluation of NVC grading quantitatively and qualitatively
Two senior radiologists blinded to the symptomatic side analyzed the MRI features of all neurovascular contacts of the bilateral trigeminal nerves in all enrolled subjects. Transverse, coronal, and sagittal planes were used to evaluate the relationship between the blood vessels and the trigeminal nerve according to previous literature assessment criteria22. The scoring criteria for NVC degree were as follows: Null point without contact; 1 point contact between nerves and blood vessels with no indentation or nerve shift; 2 points contact between nerves and blood vessels with indentation; and 3 points nerve compression due to blood vessels, resulting in distortion or displacement of nerves. A 9-point scale was used to grade nerve compression by blood vessels (NVC grading), which sums up the NVC points on transverse, sagittal, and oblique coronal planes.
Image processing
DPARSF v2.0 software based on MATLAB R2012a was used for preprocessing. Processing steps included slice timing, head-motion correction, and spatial normalization in the Montreal Neurological Institute (MNI) space. The linear trend of the fMRI data was removed. For ReHo, Kendall’s coefficient concordance (KCC) was used as an indicator to measure consistency by collecting a given voxel and those of its neighbors (26 voxels) in the resting state time series. We used Gaussian random field (GRF) theory and recorded average ReHo values in different brain regions between the two groups. On the basis of preprocessing, four-dimensional resting fMRI data were obtained by low-frequency filtering, and their time series were converted to frequency bands by a fast Fourier transform. Then, the frequency band filtering was averaged at 0.01 to 0.08 Hz, and Fisher Z-transform was performed to obtain the individual Z-transform ALFF. Two independent sample t tests were used to compare voxels, with age as a covariate.
Statistical analysis
The demographic and clinical variables of the TN and control groups were assessed using SPSS 21.0 software and an independent sample t test. P < 0.05 was considered to be statistically significant. The DPABI brain function data package (http://wiki.rfmri.org) was used to conduct two independent samples t tests for the above data, and the default mask was used in the test process23. Finally, the differential maps of ReHo and ALFF in the two groups of brain activation regions were obtained, and the number of MNI coordinates, anatomical positioning and activation intensity (T value) of the different brain regions were recorded and analyzed. We performed ALFF and ReHo analyses on both data sets with voxel level familywise error (FWE) correction (P < 0.01) and cluster level (P < 0.001). A threshold of > 18 voxels was set by AlphaSim program.
Results
Demographic and clinical characteristics
Table 1 shows the demographics and characteristics of the TN and control groups. There was no significant difference in demographic variables (sex, age, and education) between the two groups (P > 0.05). The VAS scores, duration of illness and NVC score in the TN group were 8.32 ± 1.00, 5.23 ± 6.18 years and 4.45 ± 2.85, respectively.
Brain areas with ReHo and ALFF differences between the TN and control groups
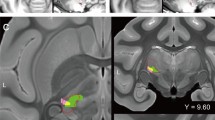
Compared to controls, brain regions with significantly enhanced ReHo values in TN included bilateral inferior cerebellum, right superior orbital frontal gyrus, right precentral gyrus, left superior cerebellum, left gyrus rectus, and left middle temporal gyrus (Fig. 1A, Table 2). Compared to controls, brain regions with significantly diminished ReHo values in the TN group included right median cingulate gyrus, right middle frontal gyrus, right supramarginal gyrus, left triangular inferior frontal gyrus, left middle temporal gyrus, and left middle frontal gyrus (Fig. 1A, Table 2). After FWE correction, the differential brain regions in ReHo values between the two groups were mainly located in the bilateral middle frontal gyrus, bilateral inferior cerebellum, right superior orbital frontal gyrus, right postcentral gyrus, left inferior temporal gyrus, left middle temporal gyrus, and left gyrus rectus (Table 2). Compared to controls, brain regions with significantly enhanced ALFF values in the TN group in right postcentral gyrus (Fig. 1B, Table 2). Compared to controls, brain regions with significantly diminished ALFF values in the TN group included left triangular inferior frontal gyrus, left supplementary motor area, left anterior cingulate, right supramarginal gyrus, and right middle frontal gyrus (Fig. 1B, Table 2). After FWE correction, the differential brain regions in ALFF values between the two groups were mainly located in the left triangular inferior frontal gyrus, left supplementary motor area, right supramarginal gyrus, and right middle frontal gyrus (Table 2).
Brain areas with ReHo and ALFF differences between the right and left affected side groups
Compared to TN patients with right-sided disease, the brain areas with significantly enhanced ReHo values in TN patients with left-sided disease included bilateral middle frontal gyrus and left precuneus (Fig. 2A, Table 2). Compared to TN patients with right-sided disease, the brain areas with significantly weakened ReHo values in TN patients with left-sided disease included left superior cerebellum and left inferior frontal gyrus (opercular part) (Fig. 2A, Table 2). After FWE correction, the differential in ReHo values between the two groups were mainly located in the right middle frontal gyrus (Table 2). Compared to TN patients with right-sided disease, the brain areas with significantly enhanced ALFF values in TN patients with left-sided disease included right middle temporal gyrus, right inferior frontal gyrus (opercular part), and bilateral superior temporal gyrus (Fig. 2B, Table 2). Compared to TN patients with right-sided disease, the brain areas with significantly weakened ALFF values in TN patients with left-sided disease included left inferior cerebellum, left middle occipital gyrus, and right superior parietal gyrus (Fig. 2B, Table 2). After FWE correction, the differential brain regions in ALFF values between the two groups were mainly located in the left middle occipital gyrus and right superior temporal gyrus (Table 2). Clinical analysis showed no significant differences in the NVC (5.39 ± 2.75 vs. 3.60 ± 2.82) and VAS scores (8.17 ± 0.99 vs. 8.45 ± 1.05) between the two groups (P > 0.05).
Brain areas with ReHo and ALFF differences between different sexes
Compared with the male TN patients, the brain areas with significantly enhanced ReHo values in female TN patients included left middle orbital frontal gyrus (Fig. 3A, Table 2). Compared with the male TN patients, the brain regions with significantly diminished ReHo values in the female TN patients included the bilateral cerebellum, bilateral inferior parietal gyrus, several frontal gyrus, left postcentral gyrus, and left middle temporal gyrus (Fig. 3A, Table 2). After FWE correction, the differential brain regions in ReHo values between the two groups were mainly located in the bilateral inferior parietal gyrus, left superior cerebellum, and right inferior frontal gyrus (opercular part) (Table 2). Compared with male TN patients, the brain regions with significantly enhanced ALFF values in female TN patients included the right superior frontal gyrus, and left middle temporal gyrus (Fig. 3B, Table 2). Compared with the male TN patients, the brain areas with significantly diminished ALFF values in female TN patients included the right occipital superior gyrus, left middle temporal gyrus, and bilateral inferior parietal gyrus (Fig. 3B, Table 2). After FWE correction, the differential brain regions in ALFF values between the two groups were mainly located in the left inferior parietal gyrus and right superior frontal gyrus (Table 2).
Brain areas with ReHo and ALFF differences between different NVC scores
Compared to low NVC scores, brain regions with ReHo values in patients with high NVC scores had no clear directivity. Compared to low NVC scores, brain areas with significantly enhanced ALFF values in patients with high NVC scores included the right precuneus and right anterior cingulate gyrus before and after FWE correction (Table 2).
Discussion
TN is a unique form of peripheral neuralgia that significantly impacts the daily functioning and productivity of individuals, yet the underlying central mechanism remains incompletely understood. In the present study, a combination of ReHo and ALFF analyses were employed to investigate abnormalities in local homogeneity and spontaneous brain activity among TN patients. ALFF quantifies the magnitude of temporal fluctuations, whereas ReHo assesses the local synchronization of neighboring voxels. Although the findings from these two methods diverge, they collectively offer valuable insights into alterations in regional spontaneous brain activity in TN patients.
DMN and the experience of pain in TN patients
Medial prefrontal region
The prefrontal cortex is implicated in many higher cognitive functions, including executive cognitive control and behavioral inhibition24. Our study revealed reduced ALFF signal in the right middle frontal gyrus and left triangular inferior frontal gyrus in TN patients compared to controls, with female TN patients also exhibiting altered ALFF signals in the right superior frontal gyrus relative to male TN patients. We hypothesize that the activation of these regions may be triggered by the electric shock-like sensations experienced in TN. Davis et al.9 found cortical thinning and loss of gray matter in patients with TN in the medial frontal and orbitofrontal cortices. These structural changes may underlie the decreased resting-state spontaneous activity observed in specific regions of the medial frontal lobe in this study. Activation of the prefrontal areas of the brain has been linked to pain regulation. At the same time, frequent and intense pain can disrupt the patient’s sleep experience, thus causing abnormalities in function in vivo and further causing frontal cortex dysfunction25. In this study, we found that compared to controls, TN patients had significant inhibition of ReHo signaling in the middle frontal gyrus bilaterally, while there was enhanced activation in the right supraorbital frontal gyrus. These findings suggest that the experience of pain and its associated functional limitations may contribute to aberrant neural functioning within frontal lobe-related regions. The enhanced brain activation observed in the right supraorbital frontal gyrus may represent a compensatory mechanism in response to these alterations. Likewise, individuals with left-sided TN exhibited increased activation in the right middle frontal gyrus relative to those with right-sided TN. When examining sex differences, female patients with TN displayed notable changes in brain activity within various regions, particularly in the right prefrontal areas, compared to male TN patients, which potentially implicating emotional control. The modifications in the medial prefrontal cortex induced by pain additionally impacted emotional regulation in females.
Parietal region
The inferior parietal lobule, comprising the supramarginal gyrus and angular gyrus, with intricate fibrous connections, is intricately linked to language function. Our study revealed that female TN patients exhibited decreased ALFF and ReHo signals in the inferior parietal lobule compared to male TN patients. This structure serves as a functional area for sensory stimulation and cognition, and females show a more robust metabolism, especially in language signal processing. The supramarginal gyrus also is also associated with emotional recognition and social interaction26,27. The precuneus is a region of heightened metabolic activity within the DMN that plays a crucial role in various higher cognitive functions and exhibits extensive functional connectivity with the frontal, parietal, temporal, and occipital cortex28,29. The precuneus is closely related to self-perception and emotional processing. In patients with TN, individuals with left-sided disease demonstrated activation of the left precuneus compared to those with right-sided disease. It can be hypothesized that frequent discharge-like stimulation in TN patients with left-sided disease caused a compensatory mechanism in the precuneus. Previous studies of TN have similarly found abnormal ReHo in the middle temporal gyrus and precuneus on brain scans30. Alterations in the precuneus and cingulate region were mainly seen in comparing patients with different degrees of NVC. Therefore, the severity of NVC remains an essential factor in judging the seriousness of TN.
Temporal lobe region and the experience of pain in TN patients
The frontotemporal lobe, the most advanced brain region, is a major component of the DMN, and is also the most structurally and functionally vulnerable region31. The anterior temporal lobe is generally associated with emotional and mental activity. In the present study, there was a significant inhibitory signal in the middle temporal gyrus in TN patients compared to controls, which, combined with data from the frontal lobe, suggests that mood and emotion regulation remain vital factors influencing these two brain regions. The supramarginal gyrus is a crucial component of the auditory speech center, mainly involved in auditory processing and sound information in concert with the angular gyrus32,33. Comparing different sexes, we found that female patients had lower signals in the inferior parietal marginal angular gyrus, which, combined with the fact that TN disease is characterized by a decline in hearing with the corners of the mouth as trigger points, could explain the results of abnormalities in brain regions related to auditory language processing.
Cerebellum and the experience of pain in TN patients
The cerebellum receives a complex source of afferent fibers, including those from the prefrontal cortex, parietal cortex, temporal cortex, and sensory-motor cortex. It also gets a wide range of somatosensory input via the spinal cerebellar pathway. The present study showed that the bilateral cerebellar lobe area II was significantly activated in TN patients. Areas I and II of the cerebellum are connected to the dorsolateral prefrontal and parietal cortex and are responsible for the association with the brain, so their activation may be related to the increased long-term and high frequency of sensory input in TN. In addition, male TN patients showed activation of left cerebellar superior regions. The activation may be related to the different pain thresholds in men and women. But, it cannot be ruled out that the activation is associated with psycho-emotional processing.
Occipital lobe and the experience of pain in TN patients
The occipital lobe is comprised of mostly visual cortex34 and is a visual processing center responsible for visual communication35. In a study in rats, the occipital lobe has recently been shown to be involved in the downstream inhibitory mechanisms of pain36. Studies on diabetic neuropathic pain (DNP) have also found a negative correlation between cortical network activity in this region and the intensity of DNP37. In this study we found that TN patients with right-sided disease had higher ALFF signals in the left middle occipital gyrus, which may be related to the activation of analgesic inhibitoury mechanisms. Although there is no evidence to elaborate on the mechanism of the role of the middle occipital gyrus in the aggregation and processing of visual information, abnormalities in the middle occipital gyrus have been found in several psychiatric disorders38,39,40. Therefore, the prolonged intrusion of TN may affect the patient’s mood and mental regulation, which in turn causes abnormalities in the metabolism of the middle occipital gyrus. Previous studies have demonstrated abnormal occipital lobe activation in patients with facial spasms41. Given the similarities between facial spasms and TN, we suspect that persistent eye socket pain may affect visual pathways, especially in those patients with TN involving V1 and characterized by orbital pain.
Sensory-motor cortex and the experience of pain in TN patients
The precentral gyrus (primary motor cortex) and the supplementary motor area are essential parts of the sensory-motor cortex and are closely related to somatic movements. In TN patients, even simple nonpainful actions can cause painful episodes, which are relieved if facial movements are restricted. Numerous studies have shown that the precentral gyrus can produce sensory pain response to repetitive TN, maxillary motor inhibition, and facial muscle tension42. The somatosensory network in healthy adults primarily encompasses bilateral precentral gyrus, postcentral gyrus, middle frontal gyrus, middle occipital gyrus, insula, and cuneus43. The postcentral gyrus serves as the central hub of this system, playing a crucial role in the integration of somatosensory and motor information44, as well as in emotion regulation45. Research indicated that individuals with depression exhibited notably reduced ALFF values in the postcentral gyrus compared to their healthy counterparts46. In the present study, TN patients showed significant activation of the right postcentral gyrus and significant inhibition of left supplementary motor area compared to controls. Enhanced functional activity in the posterior central gyrus is hypothesised to be related to compensatory mechanisms for motor deficits. In contrast, the low signal presented in the supplementary motor area may result from maladaptation that occurs in the process of regulating motor function deficit. The activation of the postcentral gyrus region in TN patients demonstrates that the degree and frequency of nociceptive episodes and the patient’s expectations of pain influence alterations in the sensory cortex to a greater extent than the restriction of facial movements.
Future directions and study limitations
Our findings should be interpreted in light of several limitations. The sample size of TN patients used in this study was limited, and some influencing factors may be confounded in subgroup analyses. For example, in the gender-subgroup analysis, since TN is highly prevalent in females, and female cases in our study far exceeded those of males, the results can only show some trends. Since the patients recruited in our study did not emphasize the initial onset, most of them had a history of taking medication. It is not clear whether the medication has some effect on brain function. TN is a chronic disease and the effect of the duration of the disease on brain function is also not known. At the same time, TN is a recurrent disease with great individual variation in inter-episode intervals and frequency of episodes, and precipitating factors, so how to calculate the course of the disease has been a controversial issue. There is no uniform standard whether the time of onset of disease should be calculated from the time of first onset or from the time of current onset. Previous studies have also shown this limitation. This is also the main reason why the disease course factor was not cited in the investigation of influencing factors in this study. Another reason is that the sample size of this study is small, which limits the grouping of TN patients by disease course. Therefore the conclusions drawn from this study need to be supported by more dimensional evidence. It is necessary to expand the sample size in future studies, especially focusing on the effects of disease duration, medications, or other treatments on brain function. In future studies, it is also necessary to refine the brain region localization of TN patients, and on this basis, further analyze the functional connectivity and topological properties of the brain, and compare the data with other peripheral neuralgia or other neurovascular compressive disorders to make the conclusions more convincing.
Conclusion
In summary, the changes in ReHo in TN patients suggest that with the functional impairment of the central pain area, the functional areas controlling memory and emotion also change during the progression of the disease. Decreased ALFF in default networks and limbic systems, as well as increased ALFF in sensory and perceptual brain regions, may reflect the abnormal influence of the continuous repetitive sensory input of TN on brain network function. TN patients with different sexes, affected sides, or degrees of nerve damage may have different central mechanisms. These results provide us with new ideas to understand and analyze the central mechanisms of TN and provides a foundation for future research.
Data availability
The data sets used and analyzed in this study are available by the corresponding authors upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia 38, 1–211. https://doi.org/10.1177/0333102417738202 (2018).
Brînzeu, A., Drogba, L. & Sindou, M. Reliability of MRI for predicting characteristics of neurovascular conflicts in trigeminal neuralgia: Implications for surgical decision making. J. Neurosurg. https://doi.org/10.3171/2017.8.JNS171222 (2018).
Hitchon, P. W. et al. Predictability of vascular conflict by MRI in trigeminal neuralgia. Clin. Neurol. Neurosurg. 182, 171–176. https://doi.org/10.1016/j.clineuro.2019.05.005 (2019).
Maarbjerg, S., Wolfram, F., Gozalov, A., Olesen, J. & Bendtsen, L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain 138(Pt 2), 311–319. https://doi.org/10.1093/brain/awu349 (2015).
Liu, J. et al. Long-term retrospective analysis of microvascular decompression in patients with recurrent trigeminal neuralgia. Front. Neurol. 11, 584224. https://doi.org/10.3389/fneur.2020.584224 (2020).
Wang, D. D. et al. Prospective comparison of long-term pain relief rates after first-time microvascular decompression and stereotactic radiosurgery for trigeminal neuralgia. J. Neurosurg. 128(1), 68–77. https://doi.org/10.3171/2016.9.JNS16149 (2018).
Wu, Q. Z. et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum. Brain Mapp. 32(8), 1290–1299. https://doi.org/10.1002/hbm.21108 (2011).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843), 150–157. https://doi.org/10.1038/35084005 (2001).
Davis, K. D. & Moayedi, M. Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 8(3), 518–534. https://doi.org/10.1007/s11481-012-9386-8 (2013).
Desouza, D. D., Moayedi, M., Chen, D. Q., Davis, K. D. & Hodaie, M. Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: A paroxysmal, sensory-triggered neuropathic pain. PloS One 8(6), e66340. https://doi.org/10.1371/journal.pone.0066340 (2013).
Obermann, M. et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. NeuroImage 74, 352–358. https://doi.org/10.1016/j.neuroimage.2013.02.029 (2013).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676 (2001).
Apkarian, A. V. et al. Chronic pain patients are impaired on an emotional decision-making task. Pain 108(1–2), 129–136. https://doi.org/10.1016/j.pain.2003.12.015 (2004).
International Association for the Study of Pain (IASP) of Accessed 2019. IASP Taxonomy. https://www.iasp-pain.org/terminology?navItemNumber=576 (2019).
Baliki, M. N., Geha, P. Y., Apkarian, A. V. & Chialvo, D. R. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 28(6), 1398–1403. https://doi.org/10.1523/JNEUROSCI.4123-07.2008 (2008).
Cheng, J. C. et al. Slow-5 dynamic functional connectivity reflects the capacity to sustain cognitive performance during pain. Neuroimage 157, 61–68. https://doi.org/10.1016/j.neuroimage.2017.06.005 (2017).
DeSouza, D. D., Davis, K. D. & Hodaie, M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain 156(6), 1112–1123. https://doi.org/10.1097/j.pain.0000000000000156 (2015).
Khan, S. A., Keaser, M. L., Meiller, T. F. & Seminowicz, D. A. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155(8), 1472–1480. https://doi.org/10.1016/j.pain.2014.04.022 (2014).
Kucyi, A. et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 34(11), 3969–3975. https://doi.org/10.1523/JNEUROSCI.5055-13.2014 (2014).
Van Petten, C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia 42(10), 1394–1413. https://doi.org/10.1016/j.neuropsychologia.2004.04.006 (2004).
Chen, Y. et al. Application of amplitude of low-frequency fluctuation to altered spontaneous neuronal activity in classical trigeminal neuralgia patients: A resting-state functional MRI study. Mol. Med. Rep. 20(2), 1707–1715. https://doi.org/10.3892/mmr.2019.10404 (2019).
Chen, M. J. et al. Preoperative evaluation of the neurovascular compression using magnetic resonance tomographic angiography: Our radiologic indications for microvascular decompression to treat trigeminal neuralgia. J. Craniofac. Surg. 25(4), e384–e388. https://doi.org/10.1097/SCS.0000000000000969 (2014).
Yan, C. G., Wang, X. D., Zuo, X. N. & Zang, Y. F. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14(3), 339–351. https://doi.org/10.1007/s12021-016-9299-4 (2016).
Wilson, C. R., Gaffan, D., Browning, P. G. & Baxter, M. G. Functional localization within the prefrontal cortex: Missing the forest for the trees?. Trends Neurosci. 33(12), 533–540. https://doi.org/10.1016/j.tins.2010.08.001 (2010).
Beebe, D. W. & Gozal, D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 11(1), 1–16. https://doi.org/10.1046/j.1365-2869.2002.00289.x (2002).
Wada, S. et al. Volume of the right supramarginal gyrus is associated with a maintenance of emotion recognition ability. PLoS One 16(7), e0254623. https://doi.org/10.1371/journal.pone.0254623 (2021).
Igelström, K. M. & Graziano, M. S. A. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia 105, 70–83. https://doi.org/10.1016/j.neuropsychologia.2017.01.001 (2017).
Tanaka, S. & Kirino, E. Functional connectivity of the precuneus in female university students with long-term musical training. Front. Hum. Neurosci. 10, 328. https://doi.org/10.3389/fnhum.2016.00328 (2016).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129(Pt 3), 564–583. https://doi.org/10.1093/brain/awl004 (2006).
Yuan, J. et al. Altered spontaneous brain activity in patients with idiopathic trigeminal neuralgia: A resting-state functional MRI study. Clin. J. Pain 34(7), 600–609. https://doi.org/10.1097/AJP.0000000000000578 (2018).
He, Y. et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132(Pt 12), 3366–3379. https://doi.org/10.1093/brain/awp089 (2009).
Sliwinska, M. W., Khadilkar, M., Campbell-Ratcliffe, J., Quevenco, F. & Devlin, J. T. Early and sustained supramarginal gyrus contributions to phonological processing. Front. Psychol. 3, 161. https://doi.org/10.3389/fpsyg.2012.00161 (2012).
Humphreys, G. F., Lambon Ralph, M. A. & Simons, J. S. A unifying account of angular gyrus contributions to episodic and semantic cognition. Trends Neurosci. 44(6), 452–463. https://doi.org/10.1016/j.tins.2021.01.006 (2021).
Machielsen, W. C., Rombouts, S. A., Barkhof, F., Scheltens, P. & Witter, M. P. FMRI of visual encoding: Reproducibility of activation. Hum. Brain Mapp. 9(3), 156–164. https://doi.org/10.1002/(sici)1097-0193(200003)9:3%3c156::aid-hbm4%3e3.0.co;2-q (2000).
Mion, M. et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain 133(11), 3256–3268. https://doi.org/10.1093/brain/awq272 (2010).
Reis, G. M. et al. Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J. Pain 11(10), 1015–1026. https://doi.org/10.1016/j.jpain.2010.01.269 (2010).
Cauda, F. et al. Altered resting state in diabetic neuropathic pain. PloS One 4(2), e4542. https://doi.org/10.1371/journal.pone.0004542 (2009).
Chen, Y. et al. The right thalamic glutamate level correlates with functional connectivity with right dorsal anterior cingulate cortex/middle occipital gyrus in unmedicated obsessive-compulsive disorder: A combined fMRI and 1H-MRS study. Aust. N. Z. J. Psychiatry 53(3), 207–218. https://doi.org/10.1177/0004867418806370 (2019).
Li, M., Xu, H. & Lu, S. Neural basis of depression related to a dominant right hemisphere: A resting-state fMRI study. Behav. Neurol. 2018, 5024520. https://doi.org/10.1155/2018/5024520 (2018).
Teng, C. et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry 18(1), 370. https://doi.org/10.1186/s12888-018-1955-9 (2018).
Haller, S. et al. Imaging of neurovascular compression syndromes: Trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. AJNR Am. J. Neuroradiol. 37(8), 1384–1392. https://doi.org/10.3174/ajnr.A4683 (2016).
Ellingson, L. D., Shields, M. R., Stegner, A. J. & Cook, D. B. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J. Pain 13(2), 195–206. https://doi.org/10.1016/j.jpain.2011.11.001 (2012).
Tomasi, D. & Volkow, N. D. Association between functional connectivity hubs and brain networks. Cereb. Cortex 21(9), 2003–2013. https://doi.org/10.1093/cercor/bhq268 (2011).
Kropf, E., Syan, S. K., Minuzzi, L. & Frey, B. N. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry 41(3), 261–269. https://doi.org/10.1590/1516-4446-2018-0183 (2019).
Zhang, S. et al. Functional connectivity of amygdala subregions predicts vulnerability to depression following the COVID-19 pandemic. J. Affect. Disord. 297, 421–429. https://doi.org/10.1016/j.jad.2021.09.107 (2022).
Liang, S. et al. Altered brain function and clinical features in patients with firstepisode, drug naive major depressive disorder: A resting-state fMRI study. Psychiatry Res. Neuroimaging 303, 111134. https://doi.org/10.1016/j.pscychresns.2020.111134 (2020).
Author information
Authors and Affiliations
Contributions
Z.W., Z.Z., and Y.L. contributed to the conception and design of the study. Z.W. acquired data, investigated, performed the statistical analysis, and wrote the original draft. Z.Z., Z.S., J.X., and Y.W. wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Zhao, Z., Song, Z. et al. Functional alterations of the brain default mode network and somatosensory system in trigeminal neuralgia. Sci Rep 14, 10205 (2024). https://doi.org/10.1038/s41598-024-60273-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60273-6
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.