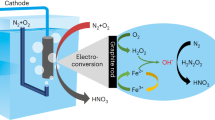
Abstract
Hydroxylamine is an important nitrogenous feedstock for the chemical industry. Conventional hydroxylamine synthesis methods utilize ammonia as the nitrogen source and require harsh reaction conditions, leading to unfavourable environmental footprint. Here we develop a plasma-electrochemical cascade pathway (PECP) powered by electricity for sustainable hydroxylamine synthesis directly from ambient air and water at mild conditions. In the first step, the plasma treatment of ambient air and water delivers a nitric acid solution with a concentration of up to 120.1 mM. Subsequently, the obtained nitric acid is selectively electroreduced to hydroxylamine using a bismuth-based catalyst. The faradaic efficiency for hydroxylamine reached 81.0% at −1.0 V versus reversible hydrogen electrode. As a result, this PECP method achieves a high hydroxylamine yield rate of 713.1 μmol cm−2 h−1 with a selectivity of 95.8%. Notably, both steps of the PECP method are operated at room temperature. Overall, our work provides a viable approach for efficient hydroxylamine synthesis from simpler feedstock at milder conditions, contributing to the sustainability transformation of the chemical industry.
Similar content being viewed by others
Main
Hydroxylamine (NH2OH), as an important nitrogenous feedstock with high reactivity, has been widely applied for the synthesis of nitrogen-containing compounds in the chemical, agrochemical and pharmaceutical fields1,2,3,4,5,6. The traditional industrial methods for the synthesis of NH2OH include the Raschig, nitric monoxide (NO) reduction and nitric acid (HNO3) reduction methods (Fig. 1a)7,8. The Raschig method employs the highly corrosive and polluting sulfur dioxide as the reductant while producing a large amount of (NH4)2SO4 as the by-product. As for both NO and HNO3 reduction methods, H2 derived from the petrochemical process and noble metal materials (Pt, Pd, Ru and Rh) have been used as proton source and catalysts, respectively, greatly increasing the cost of NH2OH production8,9,10. Although other paths such as the acetoxime method, the methyl-ethyl ketone method and the hydrogen peroxide oxidation method have been developed for the potential synthesis of NH2OH11,12,13,14,15, they have also been confronted with the high cost of reactants or the low yield of NH2OH production (Fig. 1b). Moreover, it is worth noting that the nitrogen sources for all these traditional synthetic methods for NH2OH originate from the energy-intensive Haber–Bosch process, leading to serious carbon footprint and energy consumption issues16,17,18. To circumvent the aforementioned drawbacks, it is important to exploit more practical and sustainable alternatives for NH2OH synthesis.
In this work, we developed a plasma-electrochemical cascade pathway (PECP) for renewable NH2OH electrosynthesis from ambient air and H2O (Fig. 1c). Such PECP includes the preparation of HNO3 from ambient air by plasma synthesis and the production of NH2OH in nitrate (NO3−) electroreduction. In contrast to the traditional synthetic paths, the electrosynthesis driven by renewable electricity using both green and abundant H2O as proton source paves a promising avenue for the sustainable production of NH2OH. To overcome the limitation of extremely low reactivity of the direct N2 electrochemical activation19,20,21, we exploited a home-made plasma discharge device for highly efficient N2 activation into oxynitride (NOx) by directly using ambient air as feeding gas. After steadily absorbing NOx with H2O for 30 min, we obtained an HNO3 solution with a concentration as high as 120.1 mM. Subsequently, the HNO3 solution was selectively electroreduced to NH2OH using bismuth (Bi) nanoparticles loaded on carbon fibre paper (denoted as Bi film/CFP) as the catalyst. At an applied potential of −1.0 V versus a reversible hydrogen electrode (vs RHE), Bi film/CFP exhibited a faradaic efficiency (FE) for NH2OH of 81.0%. Notably, an NH2OH yield rate of 713.1 μmol cm−2 h−1 was achieved using Bi film/CFP at −1.2 V vs RHE. Based on density functional theory (DFT) calculations, the Bi(012) facet exhibited an easier desorption for the adsorbed NH2OH intermediate (*NH2OH) relative to other metallic facets. Meanwhile, *NH2OH on Bi(012) facet showed a more difficult dissociation in comparison with these on other metallic facets, resulting in the selective formation of NH2OH in NO3− electroreduction.
Results
Plasma synthesis of HNO3 from ambient air and H2O
As indicated by the reaction mechanism and previous reports on the discharge activation of N2 (refs. 22,23), it is of vital importance to increase the collision probability between N and O radicals to improve the yield rate of NOx. Because the dissociation of inert N2 into N radicals takes place within the energy-concentrated region of the plasma discharge device, the overlapping zone between the electric arc and the ambient air is positively correlated with the activation rate of N2. Hence, we designed a plasma discharge device equipped with multiple parallel tips to enlarge the overlapping zone for the efficient activation of N2 (Supplementary Fig. 1). Typically, a high-voltage power supply with an input alternating current (AC) voltage of 50 V was adopted to generate an output AC voltage of 10 kV between the tips. To verify the feasibility of N2 plasma fixation from ambient air on the home-made plasma discharge device, we performed a qualitative analysis. When gaseous substances produced by the plasma discharge were purged into the aqueous absorbent containing 0.02 mM methyl orange, the absorbent gradually turned from light yellow to salmon pink within 30 s (Fig. 2a,b and Supplementary Video). In contrast, when ambient air was directly bubbled into the absorbent, no significant change in the colour of the absorbent was observed during the absorption time of 10 min (Supplementary Fig. 2). These results suggest that the acidic NOx produced from the plasma discharge was absorbed in aqueous solution to form HNO3.
a,b, Photographs of the pristine absorbent (a) and the absorbent after absorbing the gaseous substances produced by the plasma discharge device for 30 s (b). The vials on the left and right sides were used as control and experiment groups, respectively. c, The concentration of HNO3 in 30 ml of absorbent under various flow rates of ambient air for a 5 min discharge. d, The accumulated concentration of HNO3 in 30 ml of absorbent under the flow rate of 200 standard cm3 min−1 for various discharge times. e, Cyclic stability for the preparation of HNO3 in 30 ml of absorbent under the flow rate of 200 standard cm3 min−1 at each cycle for a 30 min continuous discharge.
To evaluate the performance for N2 fixation using the plasma discharge device, we quantified the yield of HNO3. After the absorption of gaseous products in 30 ml of H2O, the concentration of HNO3 was analysed by ion chromatography (Supplementary Fig. 3). The flow rate of the ambient air was a key parameter for optimizing the efficiency of N2 fixation. Figure 2c shows the concentration of HNO3 after the 5 min plasma discharge under various flow rates of ambient air. The concentrations of HNO3 were monotonically increased when the flow rates of ambient air were modulated from 20 to 200 standard cm3 min−1. Under the flow rate of 200 standard cm3 min−1, the concentration of HNO3 reached as high as 20.3 mM. To meet the requirement of high-yield for the preparation of HNO3, we chose the flow rate of 200 standard cm3 min−1 as the optimum condition for the continuous discharge process. As shown in Fig. 2d, the concentrations of HNO3 were almost linearly accumulated in 30 ml of absorbent after continuous discharge for 30 min. Notably, the concentration of HNO3 reached as high as 120.1 mM for 30 min. Up to date, numerous methods such as the plasma process, the ultrasound process, the electrocatalytic process and the photocatalytic process have been exploited for the direct utilization of air24,25,26,27,28. The yield rate for plasma-generated HNO3 was 7.21 mmol h−1 under the flow rate of 200 standard cm3 min−1 for a 30 min discharge. Such value is comparable to those of previous reports for air-fixation processes (Supplementary Table 1). Aside from HNO3, gaseous nitrogenous products in the outlet of the gas stream after absorption were determined by infrared detector to be NO and N2O (Supplementary Fig. 4). Although the concentration of HNO3 reached as high as 331.3 mM after a 3.5 h continuous absorption, the yield rate for HNO3 was gradually decreased during the ambient absorption process (Supplementary Fig. 5). We further conducted cyclic stability tests on the plasma discharge device. Each cycle was continuously discharged for 30 min. During 20 cyclic stability tests, the concentrations of HNO3 in the absorbent were always higher than 115.3 mM (Fig. 2e). The as-obtained aqueous HNO3 solution can be directly fed for the NO3− electroreduction process.
Catalytic performance of NO3 − electroreduction into NH2OH
Bi film/CFP was adopted as the catalyst in NO3− electroreduction. Briefly, Bi film/CFP was obtained via the magnetron sputtering of Bi disk on CFP with direct current deposition. As revealed by scanning electron microscope (SEM) measurements (Fig. 3a and Supplementary Fig. 6), Bi film was closely packed on the surface of CFP. The SEM images of the cross-sectional Bi film/CFP showed a clear interface between CFP and the Bi film layer (Supplementary Fig. 7). The magnified SEM image of the cross-sectional Bi film/CFP indicated that the thickness of the Bi film layer was ~730 nm (Fig. 3b). On the basis of SEM energy dispersive X-ray (EDX) elemental mapping analysis, the layered-stack structure was also verified by the distinct elemental distributions (Fig. 3c). As shown by high-angle annular dark field scanning transmission electron microscope (HAADF-STEM) measurement, the interplanar spacing of 0.328 nm for an individual Bi nanoparticle was assigned to the (012) facet of metallic Bi (Fig. 3d). In the X-ray diffraction (XRD) pattern, except for the characteristic peaks of CFP, the new peaks at 22.5°, 23.8°, 27.2°, 37.9°, 39.6°, 44.6°, 46.0° and 48.7° were indexed to the (003), (101), (012), (104), (110), (015), (006) and (202) facets of hexagonal Bi (JCPDS No. 41-1246), respectively (Fig. 3e). As shown by Raman spectra, the characteristic peaks at 70.5 and 97.0 cm−1 for Bi film/CFP were attributed to the Eg and A1g stretching modes of the metallic Bi-Bi bond, respectively (Fig. 3f). Supplementary Fig. 8 shows the X-ray spectroscopy (XPS) spectra. Bi film/CFP exhibited two peaks at 162.4 and 157.0 eV in the Bi 4f XPS spectrum, assigned to the 4f5/2 and 4f7/2 of Bi0 species, respectively. By contrast, no obvious signal was detected in the Bi 4f XPS spectrum of CFP.
a, SEM image of the Bi film/CFP. b,c, SEM image (b) and SEM-EDX elemental mapping images (c) of the cross-sectional Bi film/CFP. d, HAADF-STEM image of an individual Bi nanoparticle in Bi film/CFP. e,f, XRD patterns (e) and Raman spectra (f) of both Bi film/CFP and CFP. SEM imaging and HAADF-STEM imaging in a–d were repeated twice independently.
The NO3− electroreduction measurements were carried out in an H cell using 0.5 M H2SO4 and 0.1 M plasma-generated HNO3 as the catholyte. As revealed by cyclic voltammetry (CV) measurements, the current density measured in 0.5 M H2SO4 and 0.1 M plasma-generated HNO3 electrolyte was much higher than that in 0.5 M H2SO4 electrolyte, revealing the high cathodic reactivity of Bi film/CFP in NO3− electroreduction (Supplementary Fig. 9). Chronoamperometry electrolysis was conducted to quantitatively analyse the catalytic performance of NO3− electroreduction (Supplementary Fig. 10). During NO3− electroreduction, an argon (Ar) stream with a flow rate of 20 standard cm3 min−1 was used as the shielding gas to insulate the catholyte from the air. Meanwhile, the gaseous products were transported out by the Ar stream for quantification via an online gas chromatography (GC) and infrared detector. To eliminate the interference of the O–H groups in H2O with the N–H groups in NH2OH, we adopted an excess amount of glyoxylic acid (C2H2O3) to capture NH2OH via a spontaneous oximation process. As determined by 1H nuclear magnetic resonance (NMR) measurements, NH2OH was quantified on the basis of the C–H group of glyoxylic acid oxime (C2H3NO3) (Supplementary Fig. 11). In addition, other liquid products were quantitatively analysed by 1H NMR and ion chromatography (Supplementary Fig. 12). At all applied potentials from −0.6 to −1.2 V vs RHE, NH2OH was determined to be the predominant product in NO3− electroreduction using Bi film/CFP (Fig. 4a and Supplementary Fig. 13). H2 and N2O were determined to be the gaseous by-products (Supplementary Figs. 14 and 15). Notably, the FE for NH2OH using Bi film/CFP reached up to 81.0% at −1.0 V vs RHE. At −1.2 V vs RHE, the yield rate for NH2OH using Bi film/CFP was as high as 713.1 µmol cm−2 h−1 (Fig. 4b). Such value represents the highest yield rate for NH2OH among recent reports29,30,31,32 (Supplementary Table 2). In addition, the selectivity for NH2OH when using Bi film/CFP was always higher than 80.6% at all applied potentials. The highest selectivity for NH2OH of 96.6% was obtained at −1.1 V vs RHE. Isotopic labelling experiments with 15NO3− as reactants revealed that NH2OH originated from NO3− rather than any other nitrogenous contaminants (Supplementary Fig. 16). According to a series of control experiments, Bi served as the active species for the NO3− electroreduction into NH2OH (Supplementary Figs. 17–19).
a,b, FE for NH2OH (a) and the selectivity/yield rate for NH2OH (b) in NO3− electroreduction using Bi film/CFP. Experimental data were acquired via a 1 h chronoamperometry test at various applied potentials in 0.5 M H2SO4 and 0.1 M plasma-generated HNO3. Data presented as mean ± s.d. (n = 3). c, The accumulated concentration and yield rate for NH2OH during the 5 h continuous electrolysis. d, 1H NMR spectrum of the electrolyte after the 5 h continuous electrolysis with the addition of 0.2 M C2H2O3. e, Cyclic stability test in NO3− electroreduction using Bi film/CFP for each cycle for a 5 h continuous electrolysis. Inset: solid 2NH2OH·H2SO4 products separated after 12 cyclic tests. Experimental data in c and e were acquired via a 5 h chronopotentiometry test at −100 mA cm−2 in 0.5 M H2SO4 and 0.1 M plasma-generated HNO3. The corresponding data points in a are overlayed as dot plots.
To evaluate the catalytic stability of Bi film/CFP, we carried out continuous NO3− electroreduction. A 5 h continuous chronopotentiometry electrolysis at −100 mA cm−2 was first conducted to explore the accumulation of NH2OH in the catholyte. As shown in Fig. 4c, the accumulated concentrations of NH2OH were almost linearly increased, with the average yield rate of NH2OH being higher than 456.5 µmol cm−2 h−1. The accumulated concentration of NH2OH reached as high as 77.7 mM (Fig. 4d). Meanwhile, the FE and selectivity for NH2OH were always higher than 73.4% and 95.7%, respectively, during the 5 h electrolysis (Supplementary Fig. 20). We carried out cyclic stability tests to further study the catalytic stability of Bi film/CFP in NO3− electroreduction. Specifically, each cycle was continuously electrolysed at −100 mA cm−2 for 5 h (Supplementary Fig. 21). In the period of 12 cycles with a total electrolytic duration of 60 h, the product selectivity for NH2OH was still higher than 96.0% (Fig. 4e). Moreover, the average yield rate for NH2OH fluctuated within the error range of 6.0% during 12 cyclic tests. According to the analysis of double-layer capacitances derived from CV measurements, surface roughness was increased after cyclic stability tests (Supplementary Fig. 22). This result was in good agreement with the gradual increase in current density during 1 h of NO3− electroreduction. On the basis of in situ X-ray absorption fine structure (XAFS), in situ XRD and post-reaction structural characterization, the metallic Bi species in Bi film/CFP were preserved during NO3− electroreduction (Supplementary Figs. 23–28). The solid 2NH2OH·H2SO4 products (Fig. 4e inset) of 1.887 g with separation efficiency of 89.9% were achieved from the 360 ml of catholyte after 12 cyclic tests of NO3− electroreduction. Techno-economic analysis revealed that PECP for the electrosynthesis of NH2OH would be economically profitable when the electricity price is lower than US$0.027 kWh−1.
Reaction mechanism of NO3 − electroreduction into NH2OH
To unravel the intrinsic reason for the high activity and selectivity for NH2OH when using Bi film/CFP as catalyst, we investigated the reaction mechanism of NO3− electroreduction. Figure 5a shows the typical pathway for the formation of NH2OH and NH3 derived from the electroreduction of NO3−. Guided by the typical pathway, we conducted spin-polarized DFT calculations to investigate the Gibbs free energy for the formation of NH2OH and NH3 on Bi(012) facets (Supplementary Fig. 29). As shown in Fig. 5b, the potential-determining step for the formation of both NH2OH and NH3 is the protonation process of *NO into the adsorbed NHO intermediate, with variation in Gibbs free energy (ΔG) of 0.15 eV. The ΔG was calculated to be −0.73 eV for the desorption of *NH2OH into NH2OH, whereas the ΔG for the dissociation of *NH2OH into *NH2 and *OH was −0.57 eV, suggesting that the NH2OH path is thermodynamically more favourable than the NH3 path in NO3− electroreduction using Bi catalyst. Moreover, in comparison with the ΔG of 0.15 eV for the protonation of *NO, the ΔG for proton activation was as high as 1.30 eV, revealing the sluggishness of hydrogen evolution when using Bi film/CFP (Fig. 5c and Supplementary Fig. 30). These results are in accordance with the low FE for both NH3 and H2 in NO3− electroreduction using Bi film/CFP.
a, A typical reaction pathway for NH2OH and NH3 in NO3− electroreduction. b, Gibbs free energy diagram for the formation of NH2OH and NH3 on the Bi(012) facet. The red and dark cyan represent the paths for the formation of NH2OH and NH3, respectively. * indicates an adsorption site.c, Gibbs free energy diagram for hydrogen evolution on the Bi(012) facet. d, FE vs yield rate for NH2OH generated using various metallic catalysts at −1.0 V vs RHE. Experimental data were acquired via a 1 h chronoamperometry test at −1.0 V vs RHE in 0.5 M H2SO4 and 0.1 M plasma-generated HNO3. Data presented as mean ± s.d. (n = 3). e, The ΔGdiss − ΔGdes for *NH2OH on specific facets for various metals.
In general, NH3 has been reported as the final product for NO3− electroreduction using most metallic catalysts20,33,34,35. To further investigate the formation mechanism of NH2OH using Bi catalyst, we adopted various metallic materials including copper (Cu) foil, ruthenium (Ru) foil, silver (Ag) foil, platinum (Pt) foil and gold (Au) foil as cathodic catalysts in NO3− electroreduction (Supplementary Fig. 31). NH3 dominated among all the nitrogenous products for Cu foil, Ru foil and Ag foil, while H2 was detected as the main product during NO3− electroreduction using Pt foil and Au foil (Supplementary Fig. 32). Bi film/CFP exhibited both the highest FE and yield rate for NH2OH among all these catalysts (Fig. 5d). Moreover, while the Heyrovsky step served as the rate-determining step (RDS) for Pt foil, the Volmer step served as the RDS for Cu, Ru, Ag, Au and Bi catalysts (Supplementary Fig. 33). This result was in good agreement with the DFT calculation that *NO protonation served as the potential-determining step when using the Bi(012) facet. According to the structural characterizations, the exposed surfaces of the Cu foil, Ru foil, Ag foil, Pt foil and Au foil were enclosed by cubic Cu(100), hexagonal Ru(002), cubic Ag(100), cubic Pt(100) and cubic Au(100), respectively (Supplementary Fig. 34). Since the selectivity of the production of NH2OH or NH3 was determined by the behaviour of adsorbed *NH2OH, we conducted spin-polarized DFT calculations to investigate the desorption and dissociation of *NH2OH by adopting representative Cu(100), Ru(002), Ag(100), Pt(100) and Au(100) facets as model slabs (Supplementary Figs. 35 and 36). Supplementary Fig. 37 shows the ΔG of the desorption (ΔGdes) and dissociation (ΔGdiss) for *NH2OH on specific facets for various metals. The differences between ΔGdiss and ΔGdes (ΔGdiss − ΔGdes) for *NH2OH were adopted to describe the selectivity for NH2OH/NH3. A more positive value of ΔGdiss − ΔGdes for *NH2OH suggests a more favourable process for the formation of NH2OH, whereas a more negative value of ΔGdiss − ΔGdes for *NH2OH indicates a higher selectivity for NH3. Among all of the specific facets for various metals, only the Bi(012) facet exhibited a positive ΔGdiss − ΔGdes for *NH2OH (Fig. 5e). Accordingly, an easier desorption accompanied by a more difficult dissociation of *NH2OH accounted for the selective generation of NH2OH in NO3− electroreduction using Bi catalysts.
Discussion
The development of new methods for NH2OH synthesis is crucial to alleviating the heavy pollution, high cost and severe carbon emission of conventional synthetic methods. Combining plasma discharge with electroreduction processes, we implemented sustainable NH2OH electrosynthesis from ambient air and H2O. Since the proton and nitrogen sources come from abundant H2O and the air, respectively, the PECP for NH2OH synthesis holds a sustainable origin of raw materials. Integrating either oxygen evolution or anodic production of other value-added chemicals, PECP represents a sustainable green chemistry process without releasing CO2 and/or any other wastes.
Relying on renewable driving force such as wind energy, solar energy or tidal energy, air/H2O can be obtained via simply drying/purification before being put into the PECP synthesis, bringing great convenience for plant location of NH2OH production in the future. The conceptual strategy of PECP opens up a sustainable route for the renewable electrosynthesis of NH2OH, as well as other nitrogenous compounds.
Currently, lab-scale plasma synthesis of HNO3 still faces challenges such as high energy consumption and low conversion of the air. In further studies, we should pay more attention to upgrading the plasma discharge device from the perspectives of discharge types, mass transfer in the microzone of arcs, and stability of tip materials/arcs to enhance the efficiency and stability of the air-to-HNO3 process. As for the electrochemical process, optimization of both activity and energy efficiency are still urgently needed. More cost-efficient and stable catalysts should be further exploited to improve the activity, current efficiency and durability of NO3− electroreduction into NH2OH. In addition, engineering of devices, for example, the membrane electrode assembly (MEA) device, could partially eliminate electrolyte resistance and thus reduce full-cell voltage. Moreover, optimization of the flow field in the solid–liquid interface is an effective means to improve mass transfer efficiency. By advancing the channel of the MEA device and circulating the electrolyte, the concentration of NH2OH in the catholyte could be improved to reduce the energy cost of downstream separation. In addition, modulating the composition of the catholyte could realize the universal electrosynthesis of a series of hydroxylammonium salts.
Methods
Chemicals and materials
Concentrated sulfuric acid (H2SO4, 98 wt%), concentrated nitric acid (HNO3, 68 wt%), ethanol (EtOH), concentrated hydrochloric acid (HCl, 37 wt%), acetone, titanium (Ti) mesh, Cu foil, Ag foil and Pt foil were purchased from Sinopharm. Aqueous glyoxylic acid solution (C2H2O3, 50 wt%), iridium(III) chloride hexahydrate (IrCl3・3H2O) and potassium nitrate (K15NO3, 15N > 99%) were purchased from Aladdin Chemistry. Methyl sulfoxide (d6-DMSO, 99.9 atom% D), 1-propanesulfonic acid 3-(trimethylsilyl) sodium salt (DSS) and Nafion 115 membrane were purchased from Sigma-Aldrich. Ru foil, Au foil and Bi disk (99.995%, φ50.8 × 3 mm) were purchased from ZhongNuo Advanced Material. Deionized H2O was produced using a Millipore Milli-Q grade system with a resistivity of 18.2 MΩ cm. All chemicals were used as received without any further purification.
Plasma synthesis of HNO3 from ambient air and H2O
In the standard plasma synthetic process, ambient air with controllable flow rates was pumped into the home-made plasma discharge device. A high-voltage generator with input AC voltage of 50 V and power of 20 W was adopted as power supply. During the plasma synthetic process, the as-obtained NOx was absorbed by a home-made absorption tower using deionized H2O as the absorbent. The concentration of NO3− after the absorption of NOx was quantitatively analysed by ion chromatography.
Synthesis of Bi film/CFP
The Bi film/CFP was synthesized on the basis of our previous report with slight modification36. Briefly, Bi disk and CFP were used as target material and substrate, respectively. Direct-current magnetron puttering was conducted at a constant current of 50 mA under an Ar atmosphere (2.4 × 10−3 mbar, 20 standard cm3 min−1) at room temperature for 3,000 s. After the magnetron sputtering process, the as-obtained sample was directly used as the catalyst for NO3− electroreduction measurements.
Pretreatment of metallic foils
Cu foil, Ru foil, Ag foil, Pt foil and Au foil were immersed in EtOH and ultrasonically cleaned for at least 10 min. EtOH was then replaced with acetone to conduct the ultrasonic cleaning process. After three repeated treatments, the working electrodes were immersed in 10 wt% of HCl solution for 10 min to remove surface oxide. After HCl treatment, the working electrodes were washed with deionized H2O and stored in acetone for further use.
NO3 − electroreduction measurements
The NO3− electroreduction measurements were carried out in a conventional H cell separated by a Nafion 115 membrane. The anolyte was 30 ml of 0.5 M H2SO4. The 30 ml of catholyte, including 0.1 M HNO3 and 0.5 M H2SO4, was prepared by using a high concentration of plasma-generated HNO3, 98 wt% H2SO4 and deionized H2O. Bi film/CFP, IrO2-loaded Ti mesh and Ag/AgCl were used as working electrode, counter electrode and reference electrode, respectively. Chronoamperometry electrolysis was controlled by an Autolab potentiostat/galvanostat (CHI660E). All potentials were measured vs the Ag/AgCl reference electrode (vs Ag/AgCl) and converted to the reversible hydrogen electrode reference scale (vs RHE) without electrolyte resistance correction on account of the equation: E (vs RHE) = E (vs Ag/AgCl) + 0.21 V + 0.0591 × pH. During the process of NO3− electroreduction, the gaseous products in the Ar stream were monitored by an online GC and infrared detector (TH-analyser). The liquid products were detected by 1H NMR, where NH2OH was captured by an excess amount of C2H2O3 through oximation. Specifically, 0.4 ml of the catholyte after 1 h electrolysis was mixed with 0.1 ml of d6-DMSO and 12.5 µl of 50 wt% C2H2O3. DSS solution (0.1 ml, 6.0 mM) was added as an internal standard. Chronopotentiometry electrolysis at 100 mA cm−2 (corresponding to −1.0 V vs RHE) was conducted for both the continuous NO3− electroreduction and cyclic stability tests.
Calculation of FE
The FE for a specific product was calculated according to equation (1):
Cproduct is the measured concentration of a specific product (mol l−1, M); V is the volume of the electrolyte for liquid products and the total gaseous volume for gaseous products (l); N is the number of electron transfer for the formation of a specific product: 6, 8 and 2 for the formation of NH2OH, NH3 and H2, respectively; F is the Faraday constant, 96,485 C mol−1; and Q is the quantity of electric charge (coulomb, C).
Calculation of the yield rate for NH2OH
The yield rate for NH2OH was calculated on the basis of the following equation:
where n is the accumulated molar quantity during t h NO3− electroreduction (10−6 mole (μmol)); A is the geometric area of the cathodic electrode (cm2); t is the reaction time (h).
Calculation of selectivity for NH2OH
The selectivity for NH2OH was calculated according to equation (3):
where n is the accumulated molar quantity during a period of NO3− electroreduction (10−6 mole (μmol)).
Purification of 2NH2OH·H2SO4 from electrolyte
A volume of 360 ml of the electrolyte after 12 cyclic stability tests (each cycle with 30 ml of catholyte) at −100 mA cm−2 was collected, in which 15.98 g CaCl2 (corresponding to 0.4 M in 360 ml) was added into the solution. After the mixture was stirred for at least 30 min, the white precipitate (CaSO4) was removed by centrifugation. Supernatant-1 was collected and concentrated to ~10 ml at 100 °C to obtain supernatant-2. During the concentration process, HCl and HNO3 were gradually evaporated, which could be condensed for further utilization. Supernatant-2 was naturally cooled to room temperature and then stored at −5 °C overnight. The white crystalline solid was then collected by vacuum filtration and washed with cold H2O three times. The white crystalline solid was further dried at 80 °C overnight to obtain 2NH2OH·H2SO4.
Calculation of separation efficiency for solid 2NH2OH·H2SO4
Based on the quantified concentration of glyoxylic acid oxime (C2H3NO3) by 1H NMR measurements, the mass of 2NH2OH·H2SO4 was 2.099 g after 12 cyclic tests. Thus, the product separation efficiency of 2NH2OH·H2SO4 was calculated on the basis of the mass ratio of the as-obtained solid 2NH2OH·H2SO4 to the as-detected 2NH2OH·H2SO4 in the electrolyte, which is given by:
DFT calculations
Spin-polarized periodic DFT calculations were performed using the Vienna Ab-Initio Simulation Package (VASP) code at the GGA level within the PAW-PBE formalism37,38,39,40. The van der Waals interactions were described using the empirical DFT + D3 method41. The slab models of the two-layer Bi(012) facet, four-layer Cu(100) facet, three-layer Ru(002) facet, four-layer Ag(100) facet, four-layer Pt(100) facet and four-layer Au(100) facet were adopted with a vacuum of 15 Å. The total energy calculations were performed using a 3 × 3 × 1 grid and a plane wave with a cut-off energy of 400 eV. Atoms in the bottom two layers were fixed and all other atoms including adsorbates were allowed to relax until the force on each ion was smaller than 0.02 eV Å−1.
The ΔG was defined as follows:
where ΔE was obtained from the DFT calculations, ΔZPE represents the correction in zero-point energies and TΔS is the contribution of entropy41.
Instrumentation
SEM images and EDX elemental mapping images were obtained with a scanning electron microscope (SEM, JSM-6700F) operated at 5 kV. HAADF-STEM images were obtained on a JEOL ARM-200F field-emission transmission electron microscope operating at an accelerating voltage of 200 kV using Cu-based TEM grids. All SEM and HAADF-STEM imaging was repeated twice independently. XPS measurements were performed on a Kratos Axis Supra+ X-ray photoelectron spectrometer with an excitation source of Al Kα = 1,486.6 eV. The gaseous products were monitored by online gas chromatography (SHIMADZU, GC-2014). Liquid products were examined on a Varian 400 MHz NMR spectrometer (Bruker AVANCE AV III 400). The concentration of NO3− was quantitatively determined via ion chromatography (Thermo Scientific, DIONES AX-DV). Raman spectroscopy measurements were carried out on a LabRAM HR Evolution microscope (HORIBA). In situ XAFS spectra were recorded at various applied potentials during NO3− electroreduction in a home-made cell at the BL11b beamline of the Shanghai Synchrotron Radiation Facility. The transmission-mode in situ XRD measurements were performed at various applied potentials during NO3− electroreduction with Liquid Metal Jet Source (Ga-Kα: 9.24 keV, wavelength: 1.3418 Å) at the Institute of Advanced Science Facilities, Shenzhen, with Dectris PILATUS 1M detector in the 2θ range of 20–50°. The exposure time for each pattern was 5 min to get a good signal-to-noise ratio.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data in this work are available in the text and Supplementary Information. The relevant raw data for each figure or table (in the text and Supplementary Information) are listed in Excel documents and provided as source or supplementary data files. Source data are provided with this paper.
References
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Tabolin, A. A. & Ioffe, S. L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 114, 5426–5476 (2014).
Huang, H., Ji, X., Wu, W. & Jiang, H. Transition metal-catalyzed C–H functionalization of N-oxyenamine internal oxidants. Chem. Soc. Rev. 44, 1155–1171 (2015).
Zhao, F. et al. A simple and efficient approach for preparation of hydroxylamine sulfate from the acid-catalyzed hydrolysis reaction of cyclohexanone oxime. Chem. Eng. J. 272, 102–107 (2015).
Manente, F., Pietrobon, L., Ronchin, L. & Vavasori, A. Trifluoroacetic acid hydroxylamine system as organocatalyst reagent in a one-pot salt free process for the synthesis of caprolactam and amides of industrial interest. Catal. Lett. 151, 3543–3549 (2021).
Tursun, M. & Wu, C. Single transition metal atoms anchored on defective MoS2 monolayers for the electrocatalytic reduction of nitric oxide into ammonia and hydroxylamine. Inorg. Chem. 61, 17448–17458 (2022).
Tauszik, G. R. & Crocetta, P. Production of hydroxylamine from nitrogen oxide: a short review. Appl. Catal. 17, 1–21 (1985).
Benson, R. E., Cairns, T. L. & Whitman, G. M. Synthesis of hydroxylamine. J. Am. Chem. Soc. 78, 4202–4205 (1956).
Shelef, M. & Graham, G. W. Why rhodium in automotive three-way catalysts? Catal. Rev. 36, 433–457 (1994).
Murray, R. W. & Singh, M. A high yield one step synthesis of hydroxylamines. Synth. Commun. 19, 3509–3522 (1989).
Semon, W. L. The preparation of hydroxylamine hydrochloride and acetoxime. J. Am. Chem. Soc. 45, 188–190 (1923).
Mantegazza, M. A. & Leofanti, G. A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species. Stud. Surf. Sci. Catal. 82, 541–550 (1994).
Tsegaw, Y. A. et al. Formation of hydroxylamine in low-temperature interstellar model ices. J. Phys. Chem. A 121, 7477–7493 (2017).
Zecchina, A. et al. Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation. Catal. Today 32, 97–106 (1996).
Wu, P., Komatsu, T. & Yashimal, T. Ammoximation of ketones over titanium mordenite. J. Catal. 168, 400–411 (1997).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Liu, X., Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Mechanism of C–N bonds formation in electrocatalytic urea production revealed by ab initio molecular dynamics simulation. Nat. Commun. 13, 5471 (2022).
Zheng, T. et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5, 388–396 (2022).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Liu, Y. et al. A highly efficient metal-free electrocatalyst of F-doped porous carbon toward N2 electroreduction. Adv. Mater. 32, e1907690 (2020).
Winter, L. R. & Chen, J. G. N2 fixation by plasma-activated processes. Joule 5, 300–315 (2021).
Li, L. et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. 60, 14131–14137 (2021).
Li, D. et al. Direct conversion of N2 and O2: status, challenge and perspective. Natl Sci. Rev. 9, nwac042 (2022).
Ding, J. et al. Sustainable ammonia synthesis from air by the integration of plasma and electrocatalysis techniques. Inorg. Chem. Front. 10, 5762 (2023).
Kwedi-Nsah, L.-M. & Kobayashi, T. Sonochemical nitrogen fixation for the generation of NO2− and NO3− ions under high-powered ultrasound in aqueous medium. Ultrason. Sonochem. 66, 105051 (2020).
Chen, S. et al. Direct electroconversion of air to nitric acid under mild conditions. Nat. Synth. 3, 76–84 (2024).
Yu, Y. et al. A nitrogen fixation strategy to synthesize NO via the thermally-assisted photocatalytic conversion of air. J. Mater. Chem. A 8, 19623–19630 (2020).
Daems, N., Sheng, X., Alvarez-Gallego, Y., Vankelecom, I. F. J. & Pescarmona, P. P. Iron-containing N-doped carbon electrocatalysts for the cogeneration of hydroxylamine and electricity in a H2-NO fuel cell. Green Chem. 18, 1547–1559 (2016).
Sheng, X. et al. Carbon-supported iron complexes as electrocatalysts for the cogeneration of hydroxylamine and electricity in a NO-H2 fuel cell: a combined electrochemical and density functional theory study. J. Power Sources 390, 249–260 (2018).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Zhang, X. et al. Direct electro-synthesis of valuable C=N compound from NO. Chem. Catal. 2, 1807–1818 (2022).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Song, Z. et al. Efficient electroreduction of nitrate into ammonia at ultralow concentrations via an enrichment effect. Adv. Mater. 34, 2204306 (2022).
Ye, S. et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 15, 760 (2022).
Kong, X. et al. Enhancing CO2 electroreduction selectivity toward multicarbon products via tuning the local H2O/CO2 molar ratio. Nano Lett. 22, 8000–8007 (2022).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 32, 1456 (2011).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
Z.G. acknowledges the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450401), the National Natural Science Foundation of China (22322901), the CAS Project for Young Scientists in Basic Research (YSBR-022), and the National Key Research and Development Program of China (2022YFC2106000). J. Zeng acknowledges the National Key Research and Development Program of China (2021YFA1500500), the CAS Project for Young Scientists in Basic Research (YSBR-051), the National Science Fund for Distinguished Young Scholars (21925204), the National Science Fund for Distinguished Young Scholars (21925204), the National Natural Science Foundation of China (22221003, 22250007 and 22361162655), the Fundamental Research Funds for the Central Universities, the Provincial Key Research and Development Program of Anhui (202004a05020074), the Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP004), and the USTC Research Funds of the Double First-Class Initiative (YD2340002002). X.K. acknowledges the National Natural Science Foundation of China (22209163). This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication, and the Instruments Center for Physical Science, University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
J. Zeng, Z.G. and X.K. conceived the idea and co-wrote the paper. X.K. and J.N. synthesized catalysts, conducted structural characterizations and performed electroreduction tests with the help of J. Zheng and Z.X. Z.S. and X.K. designed the plasma discharge device and performed the N2 plasma fixation tests with the help of L.Q. Z.Y. performed the theoretical calculations. H.L. made suggestions on the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Chang Hyuck Choi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1 and 2, and Notes.
Supplementary Video 1
30 s absorption of NOx in the aqueous absorbent containing 0.02 mM methyl orange
Supplementary Data 1
All source data for supplementary figures.
Source data
Source Data Fig. 2
Experimental source data.
Source Data Fig. 3
Experimental source data.
Source Data Fig. 4
Experimental source data.
Source Data Fig. 5
Experimental source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, X., Ni, J., Song, Z. et al. Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway. Nat Sustain (2024). https://doi.org/10.1038/s41893-024-01330-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41893-024-01330-w