Abstract
The electrolytic reduction of CO2 in aqueous media promises a pathway for the utilization of the green house gas by converting it to base chemicals or building blocks thereof. However, the technology is currently not economically feasible, where one reason lies in insufficient reaction rates and selectivities. Current research of CO2 electrolysis is becoming aware of the importance of the local environment and reactions at the electrodes and their proximity, which can be only assessed under true catalytic conditions, i.e. by in operando techniques. In this work, multinuclear in operando NMR techniques were applied in order to investigate the evolution of the electrolyte chemistry during CO2 electrolysis. The CO2 electroreduction was performed in aqueous NaHCO3 or KHCO3 electrolytes at silver electrodes. Based on 13C and 23Na NMR studies at different magnetic fields, it was found that the dynamic equilibrium of the electrolyte salt in solution, existing as ion pairs and free ions, decelerates with increasingly negative potential. In turn, this equilibrium affects the resupply rate of CO2 to the electrolysis reaction from the electrolyte. Substantiated by relaxation measurements, a mechanism was proposed where stable ion pairs in solution catalyze the bicarbonate dehydration reaction, which may provide a new pathway for improving educt resupply during CO2 electrolysis.
Similar content being viewed by others
Introduction
At the time of its discovery in the late 19th century, CO2 electrolysis was considered a niche method to resemble the biological incorporation of carbon dioxide in plants1. However, against the backdrop of rapidly increasing carbon dioxide levels in the atmosphere, the electrochemical conversion of CO2 has assumed a new role of urgency to address the looming problems of anthropogenic climate change2,3,4. Thus, during the last decades, huge efforts have been made towards optimizing CO2 electrolysis5,6,7. One finding was that CO2 electrolysis can selectively yield products depending on the metal electrocatalyst8. At gold and silver electrodes, for instance, carbon monoxide (CO) is formed5,9. Furthermore, the product selectivity can be optimized through the electrolyte and by varying experimental conditions7,10,11. It is also established that the Hydrogen Evolution Reaction (HER) is a generally undesired process in aqueous electrolytes, which competes with the CO2 reduction reaction12,13.
Even though CO, which is one possible product of CO2 electrolysis, lacks the direct usability of, e.g., short chain alcohols, it is a highly versatile educt in the chemical industry. In the Fisher–Tropsch process, the mixture of CO and H2, also referred to as syngas, can be used for the synthesis a wide array of organic product molecules14. Moreover, CO can be easily separated from the aqueous electrolyte due to its low solubility in water. Among all catalysts yielding CO, silver is the prime choice because of its moderate cost, high selectivity and availability5,7,8,15.
The relevant thermodynamic potentials \({\phi }_{0}^{\ominus }\)vs. Normal Hydrogen Electrode (NHE) of the CO2 electrolysis at pH 7, 25 °C, 1 atm gas pressure and 1 mol L−1 bicarbonate electrolyte are2,16
Since the equilibrium potentials of CO2 reduction to CO (Eq. (1)) and the HER (Eq. (3)) are within the same range, the two reactions are expected to compete with one another. However, the electrolytic CO2 reduction undergoes an intermediate step with a high activation barrier, whereby a \({{{{{{{{{\rm{CO}}}}}}}}}_{2}}^{\bullet -}\) radical is formed (Eq. (2)). Therefore, the energy efficiency of the CO2 electrolysis, defined as the ratio of the difference in free energy between products and educts and the energy consumed in the reaction, is as low as 30–40%8. To promote the selective production of CO, specific catalysts and reaction conditions need to be chosen in order to stabilize the formation of the \({{{{{{{{{\rm{CO}}}}}}}}}_{2}}^{\bullet -}\) radical and simultaneously increase the energy barrier of the HER8,16,17.
Consequently, explicit knowledge of the chemical reactions and equilibria of CO2 in aqueous solutions is essential for advancing carbon dioxide chemistry8,18,19,20,21,22,23,24. First, gaseous CO2(g) forms an equilibrium with its solvated species CO2(aq). The carbon dioxide concentration c(CO2(aq)) in aqueous media at a partial pressure \({p}_{{{{{{{{{\rm{CO}}}}}}}}}_{2}}\) is determined by Henry’s law,
Hcp denotes the Henry solubility constant of a species for a specific solvent at a given temperature. At 10 °C, the Henry solubility is Hcp(CO2) = 5.2 × 10−4 mol m−3Pa−1, resulting in c(CO2(aq)) = 52.7 mmol at 1013 hPa CO2 partial pressure8. As CO2(aq) is in dynamic equilibrium with bicarbonate (\({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\)), two possible reaction pathways exist,
where ki/k−i are the respective forward/backward reaction rates. The first pathway comprises a two step reaction in which carbon dioxide first reacts with water to form carbonic acid (H2CO3) (Eq. (5)). In the second step carbonic acid deprotonates to form bicarbonate (Eq. (6)). This step is several orders of magnitude faster compared to the formation of carbonic acid. The second, direct pathway is a slow one-step reaction of carbon dioxide and hydroxide yielding bicarbonate (Eq. (7)). In calculations the direct path is usually neglected because it becomes relevant only at higher pH values. Finally, \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) can deprotonate further to carbonate (\({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\)):
The deprotonation of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) to \({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) is several orders of magnitude faster than the formation of bicarbonate18,20. Since acidic protons and alkaline hydroxide ions are involved for all reaction steps, the equilibrium of CO2/\({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\)/\({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) strongly depends on the pH value. Combining the equilibrium constants K1 = k1a/k−1a and K2 = k2/k−2 with \({c}_{{{{{{{{\rm{total}}}}}}}}}=c({{{{{{{{\rm{CO}}}}}}}}}_{2})+c({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-})+c({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-})\) for the sum of all involved carbon species yields the following equilibrium concentrations22,23,24,25:
In the electrolysis of aqueous CO2 solutions it was shown that only solvated CO2 is directly involved in the reduction reaction. Even though \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) cannot be electrochemically reduced, it can supply CO2 to the electrolysis via the equilibrium reaction. Thus, the pH value of the electrolyte is an essential parameter for optimizing of the electrolytic CO2 reduction. At high pH values, CO2 is only present in low concentrations. However, at low pH values, where the CO2 concentration is maximum, the HER predominates because of the high amounts of H+. Therefore, CO2 electrolysis is usually performed at an intermediate pH range of 7–9, where \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) is the prevalent species in solution and the concentration of solvated CO2 approaches its solubility limit in water. Then, \({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) contributes less than 1% of the total carbon concentration. Noteworthy here is that the electrolysis reaction creates an alkaline environment near the working electrode, whereby the pH and the concentration of carbon species near the electrocatalyst will differ from values of the bulk solution26.
While the medium pH region is optimal for the electrolytic reduction of CO2, the electrolysis reaction in aqueous media is still limited by its low solubility in water. The reaction rate of the CO2/\({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) equilibrium and the movement of bulk CO2 towards the electrode are insufficient to replenish CO2 at high current densities. To overcome this challenge, gas diffusion electrodes (GDEs) have been employed. In GDE electrolysis setups, CO2 is supplied via the gas phase into the catalytically active pores of the electrode2,6,8,27. Maximum current densities of up to 300 mA cm−2 have been reported by employing this method7.
For large scale applications of electrolytic CO2 utilization, currents starting at 400 mA cm−2 are required. This necessitates the optimization of reaction conditions particularly in electrode proximity. However, studies of reaction parameters, such as molecular mobility and chemical exchange rates, are currently limited by the lack of in operando measurement methods, i.e., methods applied under catalytic conditions of the electrolysis28,29,30. Only a limited number of in operando studies have been performed on electrolytic CO2 reduction in aqueous media, mainly using infrared and Raman spectroscopy31,32,33,34. NMR techniques and setups have only rarely been employed in this field, although the first in operando NMR study was already performed in 1975 by Richards et al.35,36,37. Most in operando NMR setups employ special cells and probes, which hinders their applicability and adaptability. Nevertheless, NMR offers an extensive tool set for investigating chemical reactions, as was demonstrated for a wide variety of catalytic, mechanistic, and kinetic studies38,39,40,41,42,43,44. The method is sensitive to the chemical environment of reaction species and can monitor changes in their molecular mobility as well as chemical exchange phenomena45,46.
As evident from the dynamic equilibrium reactions in eqs. (5) to (7), the study of chemical exchange via NMR is of particular interest for the CO2 electrolysis. For a system of two exchanging species, a NMR experiment depends on the exchange rate, i.e., the inverse of the exchange time constant Texc, and the difference in the respective resonance frequencies ∣Δν∣. If the exchange rate is significantly lower than this difference, two distinct signals are present in the NMR spectrum, and the dynamic chemical equilibrium can be qualitatively and quantitatively assessed by 1D or 2D Exchange Spectroscopy (EXSY)47,48. If the exchange rate is significantly higher than the difference in resonance frequencies, one averaged, sharp signal is observed, and exchange may be studied by relaxation experiments. In between these two boundary cases, the individual signals of the exchanging species start to broaden and merge, until eventually coalescing into one broad peak that narrows as the exchange rate increases further. For the case of equimolar concentration, coalescence takes place at an exchange rate \({r}_{{{{{{{{\rm{exc}}}}}}}}.}^{{{{{{{{\rm{coalesc}}}}}}}}}\) given by48
As evident, the coalescence point depends on ∣Δν∣ and thus the the main magnetic field strength B0, which can be exploited to identify exchange processes. Furthermore, using the modified Bloch equations developed by Gutowsky and Holm, exchange spectra can be calculated at various B0 field strengths49. By comparing these calculated spectra with observations, the experimental exchange rate can be estimated.
For the CO2 reduction reaction, two types of exchange must be considered. The first one involves the equilibrium of \({{{{{{{{\rm{CO}}}}}}}}}_{2},{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and \({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) in aqueous media. At typical electrolysis conditions, the exchange of CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) is slow enough to be studied via EXSY. The equilibrium between \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and \({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) is fast even at low temperatures, therefore an averaged carbonate peak is expected in most studies. The position of this peak may be used for in operando pH determination50,51. The second equilibrium of interest appears at high ion concentrations found in most electrolytes. Then, a solution with exclusively free, non-interacting ions can no longer be assumed, and ion pairing may take place. Several kinds of ion pairs are described in literature, which are part of a dynamic equilibrium with free ions52:
Going from left to right, the distance between the cation and anion decreases. Free ions (FI), possessing individual solvation shells, are spatially separated from their respective counter ions. In the case of solvent separated ion pairs (2SIP), the solvation shell of each distinct ion is still intact, but their respective solvent molecules directly contact one another. For solvent shared ion pairs (SIP), a single solvation layer is shared between anion and cation. Finally, there is no solvent layer between the ions of contact ion pairs (CIP), where anion and cation are in direct contact with one another. For bicarbonate electrolytes, which are frequently used in the CO2 reduction reaction, the effects of ion pairing have not been fully understood.
This work aims to eludicate how a potential dependent change in electrolyte chemistry affects the aqueous CO2 equilibria by means of in operando NMR spectroscopy. A previously published setup is used that employs a commercial NMR probe and allows for 13C NMR experiments with good sensitivity and high resolution53. The electrolytic CO2 reduction is studied at several potentials using a silver working electrode. The active species are tracked during electrolysis at two different B0 fields using 13C and 23Na NMR. Changes in molecular dynamics are assessed using longitudinal and transverse relaxation time constants. Exchange experiments are performed to determine rate constants of the dynamic equilibrium between slowly exchanging species. Eventually, these results are combined in a model that describes the potential dependence of the steady-state solvation dynamics in the electrolyte.
Results and discussion
Electrochemistry and general evolution of 13C spectra
The 13C NMR spectrum of the CO2 saturated electrolyte solution is shown in Fig. 1. It consists of a CO2 signal at 125.9 ppm and a coalesced \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}/{{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) signal at 161.8 ppm. At the present pH of 8.2 the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}/{{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) equilibrium is expected to consist 99% of bicarbonate. At the start of the in operando experiment, the solvated CO2 concentration has been determined as 55 mmol L−1, and total carbon (\({{{{{{{{\rm{CO}}}}}}}}}_{2}+{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}+{{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\)) as 1.87 mol L−1, using an external reference experiment. The shape of all 13C signals consist of a sharp peak and a downfield shoulder, which is due to B0 distortions in proximity to the metal electrodes. The sharp peak component of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) is assumed to be a coalesced signal of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) present as free ions and ion pairs, as shown later.
During the Open Circuit Voltage (OCV) stage, i.e., when no current is applied to the cell and the system is allowed to relax, the equilibrium potential drops from −31 mV to −42 mV vs. Ag/AgCl. The peak shapes of all 13C signals remain constant, and only small downfield frequency shifts in the single digit ppb range are observed during OCV. The integral of the CO2 signal, however, decreases significantly to 62% (34.1 mmol L−1) over the course of 25.6 h of OCV, which has been explained as an effect of a shifting carbonate equilibrium, and is supported by the change in equilibrium potential53.
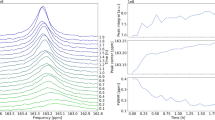
For the Chronoamperometry (CA) stage, a constant potential of − 1.1 V vs. Ag/AgCl was applied, and the evolution of current as a function of time was observed. The current density at the beginning of this stage was negative at − 12 μA cm−2, but increases quickly to values around − 4 μA cm−2 (Fig. 2 left) at ca. 35 h. This change in current density is caused by an interplay of multiple contributing factors. These factors include the depletion of the reaction educt CO2 in electrode vicinity as well as the formation of gas bubbles, which can reduce the effective electrode area upon adhesion. Another significant contribution to the evolution of the current density is the vertical orientation of the electrodes. Regions of the WE and CE closest to each other require slightly less voltage for electrolysis to take place due to internal resistance of the electrolyte solution. i.e. these regions experience a smaller iR drop. At the start of electrolysis, educt is sufficiently supplied in proximity to these low iR drop regions, and current density is considerably negative. However, after CO2 has been depleted, the electrolysis reaction shifts place to regions further apart with a higher iR drop. This causes the current density to increase to less negative values, until an equilibrium state has been reached. In addition, the current density strongly fluctuates during the whole CA experiment. These fluctuations are assumed to arise from a combination of measurement uncertainties due to the low current in the nanoampere range and increased noise levels due to the interaction of the electric leads with the RF pulses of the NMR experiment as well as bubble formation and release.
During the CA stage, the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal undergoes a transformation as the sharp peak component at 161.8 ppm splits into two components with a separation of 2.9 Hz at a magnetic field of 14.1 T (Fig. 2 middle). The peak separation is assumed to be linked to a decreasing exchange rate between free \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and the anions forming an ion pair, which is discussed in detail further below. By contrast, the CO2 signal does not change in signal shape or position, but decreases in intensity from 62 % of its original value to 37% at the end of the CA stage, i.e. the CO2 concentration decreases from 34.1 mmol L−1 to 20.4 mmol L−1 (Fig. 2 right). Assuming a faradaic efficiency of 100%, the CO2 reduction reaction consumes less than 0.28 mmol L−1 of educt. Thus the additional decrease in CO2 concentration of 13.4 mmol has to be caused by a shift of the equilibrium of CO2 in aqueous media and further a shift of local pH in the sensitive NMR volume due to the electrolysis reaction. Using the given concentrations of carbon species, a local pH of 8.4 was calculated via Eqs. (9), (11), which is in line with the amount of consumed protons or accordingly formed hydroxid given the flown charge eq. (3). This, however, implies that exchange between electrode compartments is slow at least for charged species such as OH− and H+.
For the Chronopotentiometry (CP) stage at the end, a constant current density of − 10 μA cm−2 was applied to the cell (Fig. 3 left), and the evolution of the potential versus time was recorded. At the beginning of this stage, the potential rapidly decreases to − 1.44 V vs. Ag/AgCl, and increases afterwards until it reaches an equilibrium value of − 1.41 V vs. Ag/AgCl at ca. 65 h. This overshoot of the overpotential could to be caused by turbulent micro flow currents54. Microflow currents are formed to compensate the sudden formation of density gradients in the electrolyte during the onset of the electrolysis reaction and double layer formation, and decrease in velocity after equilibration.
Time evolution of voltage and current density as well as the corresponding 13C NMR signals at B0 = 14.1 T of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and CO2 during CP with a constant current density of -10 μA cm−2. The separation between the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal components increases during this stage.
The evolution of the 13C signal during the CP stage continues the trends observed during CA. Separation between the split \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) peak components increases to 4.7 Hz at 14.1 T (Fig. 3 middle). This is due to a further decrease in exchange rate between \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) existing as free ion and in an ion pair. The peak shape and position of CO2 continue to remain unchanged, and the intensity further decreases to 22% of its original value, i.e. a concentration of 12.1 mmol L−1, at the end of the CP stage (Fig. 3 right). For the electrolysis reaction, a maximum of 0.50 mmol L−1 of the initial CO2 concentration is consumed, thus a further decrease of the CO2 concentration is likely from an increase in pH value in electrode proximity. Using the CO2 in water equilibrium reactions (Eq. (9)–Eq. (11)), the pH value at the end of CP is calculated to be 8.5. It is important to note that the pH value calculated from the CO2 concentration is an averaged value of the electrolyte inside the sensitive volume. It is a past and current point of research that a pH gradient is formed during CO2 electrolysis, which plays a significant role in catalyst activity and selectivity50,51.
Evolution of the electrolyte ion signals
As stated above, the 13C \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal splits into two peaks after OCV, which increasingly separate when an external potential is applied. The separation can be illustrated using peak deconvolution by peak fitting as shown in Fig. 4 for the 14.1 T magnetic field. This behavior is reminiscent of a system exchanging between two environments, where exchange occurs at a rate on the order of the NMR frequency differences. It is important to note that in the case of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\), the two exchanging environment must be magnetically similar as the difference in chemical shift of bicarbonate environments is small. It can also be noted that the splitting cannot be caused by a decelerated \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\)/\({{{{{{{{{\rm{CO}}}}}}}}}_{3}}^{2-}\) exchange, as the chemical shift difference between both species is significantly larger. Thus, it is proposed that the two environments are different forms of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in solution. In diluted solutions, cations and anions exist usually as free ions, i.e. an ion which is fully solvated by solvent molecules and mostly unaffected by other ions. For solutions of higher concentration, ion pairs exist in addition to free ions. Between the three types of ion pairs discussed in literature, the contact ion pair (CIP) is the most unlikely one observed in the presented 13C spectra, as the direct contact of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) with its cation is expected to have a larger effect on its electron sphere and thus the chemical shift. However, the experimental NMR data is not sufficient to distinguish between solvent shared ion pairs (SIP) and solvent separated ion pairs (2SIP); it may even be the case that both forms co-exist, with rapid exchange between them. Thus, the term solvent associated ion pairs (xSIPs) is proposed in this manuscript, where x implies one or two solvent layers, as both SIPs and 2SIPs share the feature that anion and cation are associated by solvent molecules.
The \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) evolves from the open circuit voltage (OCV) stage a over the chronoamperometry (CA) stage b to the chronopotentiometry (CP) stage c. Two signal components are assigned to \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) existing as free ions (blue) and in an ion pair (red). For high exchange rate between these two states, i.e., during OCV, the two signal components coalesce into a single peak (violet). An additional signal component (green) represents the B0 distortion in proximity to the working electrode.
The upfield component in Fig. 4b, c is assigned to the free \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) anions. The downfield component is assigned to \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) xSIPs, as the close proximity of bicarbonate to its counter cation is expected to decrease the electron density of the anion. These assignments were validated using 13C and 23Na DFT chemical shift calculations. It was found that the exact geometry of the hydration sphere of each ion in the simulation has generally a larger impact on the chemical shift than the mutual influence of anion and cation at distances larger than a contact ion pair. Thus, two hydration sphere models of varying complexity were applied. In the first model, the hydration sphere of a contact ion pair was geometry optimized. Then, the anion and cation were pulled apart with their respective hydration spheres intact, i.e. the individual ion hydration spheres are static throughout the simulations at various ion distances (Fig. S19 in the SI). Using this static hydration sphere geometry, the 13C and 23Na chemical shifts were calculated at various sodium–bicarbonate distances (Fig. S20 in the SI).
The second model included a molecular dynamics (MD) model of the hydration sphere. Here, conformational dynamics of the hydration spheres were sampled by MD simulations of the ion pairs at multiple constrained distances. For each of the samples, the 13C and 23Na chemical shifts were calculated, and their average values were computed afterwards (Fig. S21 and Table S1 in the SI).
Both models are in agreement with the peak assignment both of the 13C spectra and the 23Na spectra, which are presented later in the text. It can be highlighted that the simulations not only predict the direction of the shift in chemical shift correctly, but also the order of magnitude, although no further statement on the presence of 2SIPs versus SIPs can be given. Further discussion on the chemical shift calculations are given in the supplementary methods section in the SI.
In the experimental spectra, free ion and xSIPs signals are separated when the exchange rate between these ion forms is slower than the coalescence point as described in Eq. (12), and they are coalesced into a single peak (Fig. 4a) if it is faster. As evident from the evolution of the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal, the exchange rate is fast under OCV conditions, and continuously decreases when an increasingly negative potential is applied. Thus, it can be stated that stable electrolyte ions and ion pairs, i.e. species with a long life time, are formed when a potential is applied to the system.
The presence of two exchanging environments for the electrolyte ions can be further verified by two experiments. First, the 13C in operando spectra can be recorded at a lower magnetic field strength. The coalescence point is dependent on the B0 field, as according to Eq. (12) it is a function of the absolute peak separation in Hz, and not the relative one in ppm. Thus, under otherwise identical conditions, a lower peak separation of the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal is expected at lower field strength. This is proven true as evident in Fig. 5. Analogous to the in operando experiments at 14.1 T, one single, coalesced \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) is observed at OCV (Fig. 5a), which splits up into free ion and xSIP signal for the CA and CP stage. In accordance with the predictions, the peak separation is only 1.0 Hz (Fig. 5b) and 1.4 Hz (Fig. 5c) at the end of the CA and CP stage, respectively, and thus significantly lower compared to the experiment at 14.1 T.
The \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) evolves from the open circuit voltage (OCV) stage a over the chronoamperometry (CA) stage b to the chronopotentiometry (CP) stage c in a similar fashion to Fig. 4, where the free ion are depicted in blue, ion pairs in red, and coalesced signal in purple. An additional signal component (green) represents the B0 distortion in proximity to the working electrode. Due to the weaker B0 field strength, the separation of the free ion and ion pair signal is not as pronounced.
Furthermore, based on the experimental data at 14.1 T, exchange spectra at 9.4 T can also be calculated using the modified Bloch equations given in the work of Williams et al.49. By comparing these calculations with the observed spectra at 9.4 T, the global exchange rate of the system can be estimated to be 15 s−1. This is the minimum exchange rate at CP, and increases during the CA and OCV stage. The global exchange rate is the inverse of the inverse exchange time, a combined value of forward and backward exchange times τglobal, which is defined as τglobal = τforward ⋅ τbackward/(τforward + τbackward).
For the second verification experiment, in operando spectra were recorded for the counter ion, which is potassium or sodium, depending on the bicarbonate salt used. As 23Na has a significantly higher NMR sensitivity compared to 39K, 23Na in operando experiments were conducted. Due to the inherently broader lines of 23Na caused by its short T2 time constants, the evolution of the sodium cation signal is not as obvious, but observable nonetheless (Fig. 6). The component originating from the B0 distortion cannot be distinguished, thus the 23Na signal consists of a signal peak at OCV. At the CA stage, the formation of a shoulder can be clearly observed, which becomes more pronounced during the CP stage. A precise value for the peak separation cannot be reliably determined for 23Na, as the peak deconvolution is challenging given the high line widths. However, the peak separation can be estimated to be higher than 6 Hz, which differs significantly from the peak separation for the 13C \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal. Thus, it can be safely established that the evolution of the electrolyte signals is not due to distortion of the B0 field, but must be induced by physico-chemical processes taking place in the electrolyte solution.
The Na+ cation signal evolves from a coalesced state (purple) in the open circuit voltage (OCV) stage a over the chronoampoerometry (CA) stage b to the chronopotentiometry (CP) stage c by splitting into the two components of free ions (blue) and ion pairs (red), similar to the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal in Fig. 4. However, due to the inherently broader line widths of 23Na, the peak splitting is not as pronounced and the B0 distortion artifact cannot be observed separately.
Effects on electrolyte chemistry
The effect of the formation of stable free ions and ion pairs can be studied by investigation of NMR relaxation times as well as the exchange rate of the dynamic equilibrium between CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in aqueous solution. As presented in Table 1, it is obvious that the \({{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) exchange rate increases during the CA and CP stage. Therefore, the \({{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) equilibrium reaction accelerates when the exchange between free ions and xSIPs slows down, and more stable solvated ions and ion pairs are formed.
The origin of this effect can be explained based on the longitudinal (T1) NMR relaxation time constants presented in Fig. 7 and Table 2. Here, after the coalesced \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) peak splits into two components during the CA stage, T1 of free ions and xSIPs differ significantly, and the gap increases during the CP stage. T1 of the xSIPs is lower than of the free ions, which hints towards a longer correlation time and thus lower mobility of the xSIPs according to Bloembergen-Purcell-Pound (BPP) relaxation theory55. This is apparent, as an ion pair of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and Na+ has a larger inert mass and hydrodynamic radius compared to a free anion or cation. In addition, T1 of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in xSIPs may be affected by the quadrupolar moment of sodium or potassium due to their close proximity. An analogous effect can be observed for the transverse (T2) NMR relaxation time constants of the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) components.
At the chronoamperometry (CA) stage, the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal splits into two components for which different relaxation time constants for free ions and xSIPs can be distinguished. With increasingly negative potential at the CA and chronopotentiometry (CP) stage, CO2 approaches the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) xSIPs in terms of T1. The error bars represent the fitting error received for T1 determination. The term xSIP represents ion pairs with one (x = 1) or two (x = 2) solvent layers between anion and cation.
The relaxation time constants T1 and T2 of CO2 also surprisingly decrease as a function of the applied potential. This cannot be an effect of the CO2 reduction reaction, as only a small percentage of CO2 reacts during each stage. Furthermore, only CO2 in electrode proximity should be affected; NMR, however, measures the bulk of the aqueous electrolyte solution. Instead, T1 of CO2 seems to approach that of the xSIP \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) species due to the increasing exchange rate between CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\). This is especially apparent for the CP stage, where the relaxation time constants of CO2 fall below that of free \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\). The observation implies that the dynamic equilibrium of CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in aqueous solution preferentially takes place with \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in ion pairs, and to a lesser extent with free bicarbonate. In combination with the observation of increased \({{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) exchange rates, this suggests a model where the xSIP of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and its cation catalyzes the (de-)hydration of CO2 in aqueous solution. A description of the catalyzed equilibrium reaction in case of a sodium cation is depicted in Fig. 8. Here, the cation stabilizes the negative charge distribution at two oxygen atoms of CO2 or \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\), and thus aids the addition or elimination of a hydroxide group. As a sufficient life time of the catalyst is critical for the completion of the catalytic cycle, its efficiency increases as the exchange rate between free ions and xSIPs decreases, i.e. the xSIPs become more stable. A similar mechanism has been found for the carbonic anhydrase enzyme, which regulates the pH value in human bodies. There, the catalytic center is a zinc cation that binds to the oxygen atoms of CO2 or \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\). The complex and specialized nature of an enzyme enables significantly higher efficiency and reaction rates. However, the catalytic reactions remains analogous.
To conclude, a catalytic activity of the electrolyte cation for the CO2/\({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) equilibrium reaction is suggested based on the evolution of relaxation and exchange times akin to enzymatic systems found in nature. The catalytic activity increases with the life time of the cation in a xSIP, which is inversely proportional to the exchange rate between xSIPs and free ions (Fig. 9).
Evolution and correlation of the CO2/\({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) equilibrium reaction rate (\({k}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}}\)) and the exchange rate between free electrolyte ions and xSIPs (\({k}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{\rm{FI}}}}}}}}/{{{{{{{\rm{IP}}}}}}}}}\)) as a function of potential. With increasingly negative potential, it was found that \({k}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{\rm{FI}}}}}}}}/{{{{{{{\rm{IP}}}}}}}}}\) decreases, while \({k}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}}\) increases simultaneously. Using T1 relaxation time experiments, it was shown that CO2 preferably is formed from \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) that is composing a xSIP, which suggests a catalytic activity of the electrolyte cation, e.g. Na+. The term xSIP represents ion pairs with one (x = 1) or two (x = 2) solvent layers between anion and cation.
As a last note, the relaxation time constants of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) for the component which experiences B0 distortion reveal that the dynamics of the electrolyte volume in electrode proximity differ significantly from the bulk solution. This is especially noticeable for T1, which is identical at OCV for the bulk and electrode proximate signal components. Only when a potential is applied at the CA and CP stages, the relaxation times change from the values found in the bulk electrolyte. These changes are unlikely caused by B0 and B1 field distortions, as the relaxation times are not sensitive to ppm changes in the main magnetic field, and the saturation recovery and CPMG NMR pulse sequences used here are assumed to be robust in terms of B1 homogeneity. Further investigations of the electrolyte regions close to the electrocatalyst are currently ongoing.
Origin of the electrolyte ion separation
Upon application of an external potential, the changes to the electrolyte chemistry described here represent a separation of electrolyte ions into ion pairs and free ions. This effect can be interpreted as a consequence of Le Chatelier’s law. Upon application of the potential, an electric field is generated between the electrodes. The charged electrodes attract cations or anions from the solution in order to form a double layer. Thus, the local charge density of the electrolyte increases in proximity to the electrodes, which is counteracted by two mechanisms. First, the shielding of the ions by the formation of isolating solvent shells is more pronounced, which leads to the formation of stable free ions. Secondly, stable ion pairs are formed to combat the concentration gradient and charge separation taking place in the solution. As a result of both mechanisms, the exchange rate between the stabilized free ions and ion pairs is decreased.
A significant consequence of the observations is that the electric field applied during electrolysis affects the bulk of the electrolyte. Otherwise, only minuscule observations would be made, as NMR is an analytical method with bulk sensitivity. This notion is opposed to the classical models in electrochemistry, e.g. the Stern model of the electrical double layer, where the effect of the applied potential only extends up to a few nanometers from the electrodes into the electrolyte, and can be approximated by the Debye length. This observation is consistent with a recent publication of Zhang et al., which also suggests that the chemical impact of the electric field in electrolysis may extend significantly into the bulk of the electrolyte56.
Conclusion
In operando13C and 23Na NMR was applied to follow the major evolution taking place for the electrolyte anion bicarbonate and the respective cation during electrolytic CO2 reduction. As a function of increasingly negative potential, the exchange rate between free electrolyte ions and electrolyte solvent associated ion pairs (xSIPs) decreased. As a result, distinct chemical environments with longer lifetime were formed for both \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and its counter ion. The formation of xSIPs with a long lifetime may accelerate the equilibration reaction between \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) and CO2, which promotes the resupply of CO2 during the electrolytic reaction based on a mechanism were the cation stabilizes the the angled structure and negative charge of \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) during deprotonation.
Finally, the change in electrolyte chemistry may impact the CO2 reduction reaction. The electrolytic conversion of CO2 is limited by the low solubility in water. Multiple approaches have been studied to overcome this limitation, of which the most successful one has been gas diffusion electrodes. But at current densities >300 mA cm−2, as required for an industrial application of electrolytic CO2 reduction, the solubility of CO2 becomes again a limiting factor. In theory, most electrolytes have stored a surplus of CO2 as \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\). However the exchange reaction of the dynamic equilibrium is too low to be feasible for CO2 resupply even at medium current densities. This study, however, suggests that the exchange rate can be manipulated for electrolysis application, e.g. by tuning the catalytic properties of the electrolyte cations. Further research in this field may lead to the design of special electrolyte cations or co-catalysts that enable \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) in the electrolyte to become a more effective supplier of CO2 and thereby enable higher current densities for the CO2 reduction reaction.
Methods
In operando electrolysis setup
13C and 23Na NMR was employed in order to evaluate all the species present during the electrolysis experiments. For 13C in operando NMR measurements, an 1 mol L−1 aqueous solution of 98% 13C enriched KHCO3 (Sigma Aldrich, Munich, Germany) was used as electrolyte. Potassium salt electrolytes are beneficial for the electrolysis performance due to the size of the cation, but the stable potassium nuclei possess a low sensitivity in NMR spectroscopy. Thus 23Na experiments were performed for the investigation of the electrolyte cation using an 1 mol L−1 aqueous solution of 99.9% pure NaHCO3 (Sigma Aldrich, Munich, Germany) as electrolyte. The electrolyte was pre-chilled inside a polyethylene vial in a 10 ∘C water bath. Ca. 1 mL of chilled electrolyte was filled into a 5 mm NMR tube and bubbled with 99% 13C enriched CO2 (Cambridge Isotope Laboratories, Tewksbury, USA) using a 1/16 inch PEEK tube at a flow rate of ca. 0.3 mL s−1 and a temperature of 10 ∘C. Afterwards, the electrode setup was placed inside the 5 mm tube with CO2 saturated electrolyte. The gas phase was aerated with 13C labeled CO2 gas prior to sealing the cell. All preparation steps were performed at ambient conditions.
The electrolysis cell consisted of a three-electrode setup with a silver plate working electrode (GoodFellow, Hamburg, Germany), an Ag/AgCl micro reference electrode made in-house, and a carbon counter electrode (GoodFellow, Hamburg, Germany). The three-electrode setup was inserted into a 5 mm NMR tube. The cell was placed in a commercial NMR probe and connected to a BioLogic SP-200 potentiostat (BioLogic Science Instruments, Seyssinet-Pariset, France). Additionally, shielding and noise reduction equipment were required to achieve serviceable signal-to-noise ratios for NMR experiments. The setup employed in this study is described in more detail in our previously published work53.
General NMR experimental procedure
13C NMR experiments were performed using either a Bruker Avance III HD spectrometer equipped with a 14.1 T magnet (150.9 MHz 13C frequency) or with a 9.4 T magnet (100.6 MHz 13C frequency). Both spectrometers were equipped with double resonance broad band probe heads. 23Na studies were conducted on the 14.1 T spectrometer (158.7 MHz resonance frequency). All NMR experiments were performed at a sample temperature of 10 ∘C. All magnets were shimmed until linewidths of ca. 1 Hz were achieved for the 13C measurements or ca. 30 Hz for 23Na. For the 13C experiments at 14.1 T, a 90∘ pulses length of 15.5 μs at a pulse power of 58.7 W with a relaxation delay of 85 s was set. For the 13C experiments at 9.4 T, a 90∘ pulses length of 14.0 μs at a pulse power of 39.0 W with a relaxation delay of 85 s was set. For 23Na experiments at 14.1 T, 90∘ pulses with a length of 16.5 μs and pulse power of 40.0 W, and a relaxation delay of 1 s were used.
In operando experimental procedure
For both the 13C and 23Na studies, the in operando investigations were divided into three stages. Due to different experiment duration for each nucleus, the total time of each stage was 25.6 h for 13C and 12.3 h for 23Na investigations. During the first stage, the system was allowed to relax at open circuit voltage (OCV). During the second stage, chronoamperometry (CA) was performed by applying a potential of − 1.1 V vs. Ag/AgCl to the working electrode. During the third stage, chronopotentiometry (CP) was conducted, where a current of − 1 μA was applied for the 13C experiments resulting in a specific current density of − 10 μA cm−2. The change to a sodium electrolyte for the the 23Na investigations affects the CO2 electrolysis. Thus, the current density was adjusted in order to obtain similar potentials compared to the 13C experiments with potassium electrolyte. Low current densities were employed during the electrochemical experiments to reduce the formation rate of CO and H2 gas, which can disturb the electrochemical and the NMR measurements.
At the beginning of each stage, the system was allowed to settle in for 12 h while spectra were recorded continuously using a time step of 6 min for a total of 120 spectra. Full 13C and 23Na spectra during OCV, CA, and CP stage for each set of experiments at a time resolution of 1 hour are presented in the SI figs. S1–S9. For the 13C experiments, the CO2 saturated electrolyte was monitored by determining the longitudinal relaxation time constant T1, the transverse relaxation time constant T2, and the exchange time \({T}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}}\) between solvated CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) after recording the spectra. T1 was determined using saturation recovery with a train of equispaced saturation pulses and logarithmically spaced recovery times between 1 s and 128 s. For T2 determination a Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence with an echo time of 5 ms was employed57,58. The chemical exchange between CO2 and \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) was assessed by 1D exchange spectroscopy (EXSY) using a selective inversion of the \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) resonance by means of a Gauss shaped pulse with 100 Hz excitation bandwidth59. The chemical shifts were referenced to sodium trimethylsilylpropanesulfonate (DSS) (Sigma Aldrich, Munich, Germany) by means of an external measurement. The in operando experiments did not include DSS as the salt can interfere with the electrochemical measurements.
The 23Na experiments were conducted in a similar manner, where, first, spectra were recorded for a total of 12 h with a time resolution of 6 min. Afterwards, T1 and T2 were determined using saturation recovery and CPMG experiments, respectively. T1 was determined using saturation recovery with a train of equispaced saturation pulses and logarithmically spaced recovery times between 0.01 s and 0.96 s. For T2 determination a CPMG pulse sequence with an echo time of 0.4 ms was employed57,58. The chemical shift was not referenced to any standard as the 23Na experiments were used for validation purposes only.
NMR data evaluation
For post processing, a 1 Hz line broadening was applied to all 13C spectra, and no line broadening was applied to the 23Na spectra. NMR signals were deconvoluted by peak fitting using LMFIT version 0.9.14 for Python60, employing a non-linear least-squares algorithm. The 13C \({{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}\) signal was fitted using two (OCV stage) or three (CA and CP stage) Lorentz peaks. For 23Na, one (OCV stage) or two (CA and CP stage) Lorentz peaks were fitted due to the limited resolution given by the inherently broad lines of the spin-3/2 resonances. As a note, the stability of the fit depended strongly on the number of free fit parameters and the overlap of the individual components. In case of a single spectrum the fit with either a large number of fit parameters and moderate overlap of the resonances, as for 13C NMR, or a moderate number of parameters and a large overlap, as for 23Na NMR, may lead to unstable fit results. However, due to the large number of spectra and a continuous time evolution of the signal components, the stability of the fit procedure was significantly improved by correlation of the individual fits within a time series.
The determination of relaxation time constants, and evaluation of the saturation recovery and CPMG experiments is depicted in the SI figs. S10–S13 and figs. S14–S17, respectively. For the evaluation of the EXSY experiments, \({T}_{{{{{{{{\rm{exc}}}}}}}}}^{{{{{{{{{\rm{CO}}}}}}}}}_{2}/{{{{{{{{{\rm{HCO}}}}}}}}}_{3}}^{-}}\) was determined by fitting the evolution of the CO2 signal integral I(CO2) as a function of the mixing time τm to
where I0 is the signal integral at τm = 0. This equation is valid under the conditions that the bicarbonate concentration exceeds the CO2 concentration and that both species possess similar T1 time constants59. The evaluation of the EXSY experiments is shown in more detail in the SI Fig. S18.
DFT chemical shift calculations
Density functional theory (DFT) based geometry optimisations and chemical shift calculations were conducted using ORCA version 5.0.2.61 Geometry optimisation was done at B3LYP level with def2-TZVP62 basis set, Grimme’s D3 dispersion correction63 and Resolution of Identity approximation. The solvent was modelled using a conductor-like polarisable continuum model (CPCM) for water and by explicitly including 5 water molecules each for the bicarbonate and the sodium ion. Chemical shift calculations were done with the same parameters with automatic generation of auxiliary basis sets64. Ab initio molecular dynamics (AIMD) simulations were done using the ORCA-MD module with identical functional and basis sets. Initial structures for the AIMD simulations were obtained by geometry optimising ion pair structures with specific distance constraints between Na and C atoms. The distance constraint was also applied throughout the AIMD trajectory. Velocities were initialised for a temperature of 298.15 K and maintained using a Nosé-Hoover thermostat with a time constant of 10 fs. Each MD trajectory was 1000 fs long with a time step of 1 fs. Chemical shift calculations were done for ion pair structures extracted from AIMD snapshots at intervals of 5 fs. Additional details of the chemical shift calculations are discussed in the supplementary methods in the SI.
Data availability
NMR data are available for download at https://doi.org/10.26165/JUELICH-DATA/0GQJFC. Other data are available from the authors upon request.
Change history
01 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s42004-024-01156-9
References
Fenton, H. J. H. LXIV.–the reduction of carbon dioxide to formaldehyde in aqueous solution. J. Chem. Soc. Trans. 91, 687–693 (1907).
Zhu, D. D., Liu, J. L. & Qiao, S. Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 28, 3423–52 (2016).
Whipple, D. T. & Kenis, P. J. A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451–3458 (2010).
Jhong, H. R., Ma, S. C. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191–199 (2013).
Jeanty, P. et al. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO2 Utilization 24, 454–462 (2018).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2018).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes, chap. 3, 89–182. Modern Aspects of Electrochemistry (Springer, New York, 2008), 42 edn.
Hori, Y., Murata, A., Kikuchi, K. & Suzuki, S. Electrochemical reduction of carbon dioxides to carbon monoxide at a gold electrode in aqueous potassium hydrogen carbonate. J. Chem. Soc. Chem. Commun. (1987).
Thorson, M. R., Siil, K. I. & Kenis, P. J. A. Effect of cations on the electrochemical conversion of CO2 to CO. J. Electrochem. Soc. 160, F69–F74 (2012).
Chaplin, R. P. S. & Wragg, A. A. Effects of process conditions and electrode material on reaction pathways for carbon dioxide electroreduction with particular reference to formate formation. J. Appl. Electrochem. 33, 1107–1123 (2003).
Murata, A. & Hori, Y. Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. Bull. Chem. Soc. Jpn 64, 123–127 (1991).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal-electrodes in aqueous-media. Electrochim. Acta 39, 1833–1839 (1994).
Dry, M. E. The Fischer–Tropsch process: 1950–2000. Catal. Today 71, 227–241 (2002).
Lu, Q. et al. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 5, 3242 (2014).
Schneider, J., Jia, H., Muckerman, J. T. & Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 41, 2036–51 (2012).
Kortlever, R., Shen, J., Schouten, K. J., Calle-Vallejo, F. & Koper, M. T. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–82 (2015).
Wang, X., Conway, W., Burns, R., McCann, N. & Maeder, M. Comprehensive study of the hydration and dehydration reactions of carbon dioxide in aqueous solution. J. Phys. Chem. A 114, 1734–40 (2010).
Xiang, Q., Fang, M., Yu, H. & Maeder, M. Kinetics of the reversible reaction of CO2(aq) and \({{{\mathrm{HCO}}}}_{3}^{-}\) with sarcosine salt in aqueous solution. J. Phys. Chem. A 116, 10276–84 (2012).
Gibbons, B. H. & Edsall, J. T. Rate of hydration of carbon dioxide and dehydration of carbonic acid at 25∘C. J. Biol. Chem. 288, 3502–3507 (1963).
Toan, S. et al. Thermodynamics of NaHCO3 decomposition during Na2CO3-based CO2 capture. J. Environ. Sci. (China) 78, 74–80 (2019).
Stumm, W. & Morgen, J. J.Aquatic Chemistry : Chemical Equilibria and Rates in Natural Waters (John Wiley & Sons Inc, New York, US, 1995), 3 edn.
Harned, H. S. & Scholes, S. R. The ionization constant of \({{{\mathrm{HCO}}}}_{3}^{-}\) from 0 to 50∘. J. Am. Chem. Soc. 63, 1706–1709 (1941).
Harned, H. S. & Davis, R. The ionization constant of carbonic acid in water and the solubility of carbon dioxide in water and aqueous salt solutions from 0 to 50∘. J. Am. Chem. Soc. 65, 2030–2037 (1943).
Millero, F. J., Graham, T. B., Huang, F., Bustos-Serrano, H. & Pierrot, D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 100, 80–94 (2006).
Gupta, N., Gattrell, M. & MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 36, 161–172 (2005).
Burdyny, T. & Smith, W. A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 12, 1442–1453 (2019).
Bañares, M. A. Operando methodology: combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 100, 71–77 (2005).
Weckhuysen, B. M. Determining the active site in a catalytic process: operando spectroscopy is more than a buzzword. Phys. Chem. Chem. Phys. 5, 4351–4360 (2003).
Moraes, I. R., Kalbáč, M., Dmitrieva, E. & Dunsch, L. Charging of self-doped poly (anilineboronic acid) films studied by in situ ESR/UV/Vis/NIR spectroelectrochemistry and ex situ FTIR spectroscopy. ChemPhysChem 12, 2920–2924 (2011).
Dunwell, M., Yan, Y. & Xu, B. In situ infrared spectroscopic investigations of pyridine-mediated CO2 reduction on Pt electrocatalysts. ACS Catal. 7, 5410–5419 (2017).
Firet, N. J. & Smith, W. A. Probing the reaction mechanism of CO2 electroreduction over Ag films via operando infrared spectroscopy. ACS Catal. 7, 606–612 (2016).
Deng, Y. & Yeo, B. S. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando Raman spectroscopy. ACS Catal. 7, 7873–7889 (2017).
Dutta, A., Morstein, C. E., Rahaman, M., Cedeño López, A. & Broekmann, P. Beyond copper in CO2 electrolysis: effective hydrocarbon production on silver-nanofoam catalysts. ACS Catal. 8, 8357–8368 (2018).
Richards, J. A. & Evans, D. H. Flow cell for electrolysis within the probe of a nuclear magnetic resonance spectrometer. Anal. Chem. 47, 964–966 (1975).
Bussy, U. & Boujtita, M. Review of advances in coupling electrochemistry and liquid state NMR. Talanta 136, 155–60 (2015).
Falck, D. & Niessen, W. M. A. Solution-phase electrochemistry-nuclear magnetic resonance of small organic molecules. TrAC Trends Anal. Chem. 70, 31–39 (2015).
Abbott, T. M., Buchanan, G. W., Kruus, P. & Lee, K. C. 13C nuclear magnetic resonance and raman investigations of aqueous carbon dioxide systems. Can. J. Chem. 60, 1000–1006 (1982).
Boisseau, R., Bussy, U., Giraudeau, P. & Boujtita, M. In situ ultrafast 2D NMR spectroelectrochemistry for real-time monitoring of redox reactions. Anal. Chem. 87, 372–5 (2015).
Hall, A. M. R. et al. Practical aspects of real-time reaction monitoring using multi-nuclear high resolution FlowNMR spectroscopy. Catal. Sci. Technol. 6, 8406–8417 (2016).
Mani, F., Peruzzini, M. & Stoppioni, P. CO2 absorption by aqueous nh3 solutions: speciation of ammonium carbamate, bicarbonate and carbonate by a 13C NMR study. Green. Chem. 8, 995–1000 (2006).
Moret, S., Dyson, P. J. & Laurenczy, G. Direct, in situ determination of pH and solute concentrations in formic acid dehydrogenation and CO2 hydrogenation in pressurised aqueous solutions using 1H and 13C NMR spectroscopy. Dalton Trans. 42, 4353–6 (2013).
Perinu, C., Arstad, B. & Jens, K.-J. 13c NMR experiments and methods used to investigate amine-CO2-H2O systems. Energy Procedia 37, 7310–7317 (2013).
Seravalli, J. & Ragsdale, S. W. 13C NMR characterization of an exchange reaction between CO and CO2 catalyzed by carbon monoxide dehydrogenase. Biochemistry 47, 6770–81 (2008).
Keeler, J. Understanding NMR Spectroscopy, chap. 7, 1-29 (John Wiley and Sons, Chichester, U.K., 2010), 2 edn.
Levitt, M. H.Spin Dynamics: Basics of Nuclear Magnetic Resonance, chap. 19, 507–526 (Wiley, Chichester, 2008), 2 edn.
Binsch, G. Unified theory of exchange effects on nuclear magnetic resonance line shapes. J. Am. Chem. Soc. 91, 1304–1309 (1969).
Green, M. L., Wong, L. L. & Sella, A. Relationship between intramolecular chemical exchange and NMR-observed rate constants. Organometallics 11, 2660–2668 (1992).
Williams, K. C. & Brown, T. L. Organometallic exchange reactions. ii. lithium-7 and proton nuclear magnetic resonance spectra of alkyllithium and lithium tetraalkylmetalate solutions in ether1. J. Am. Chem. Soc. 88, 4134–4140 (1966).
Schatz, M., Jovanovic, S., Eichel, R.-A. & Granwehr, J. Quantifying local pH changes in carbonate electrolyte during copper-catalysed CO2 electroreduction using in operando 13C NMR. Sci. Rep. 12, 8274 (2022).
Schatz, M., Kochs, J., Jovanovic, S., Eichel, R.-A. & Granwehr, J. In operando magnetic resonance imaging reveals local ph and ion concentration profiles during cu-catalyzed CO2 electroreduction. ECS Meet. Abstr. MA2023-01, 2498 (2023).
Buchner, R., Chen, T. & Hefter, G. Complexity in “simple” electrolyte solutions: Ion pairing in MgSO4(aq). J. Phys. Chem. B 108, 2365–2375 (2004).
Jovanovic, S. et al. An electrochemical cell for in operando 13C nuclear magnetic resonance investigations of carbon dioxide/carbonate processes in aqueous solution. Magn. Reson. 2, 265–280 (2021).
Hamann, C. H., Hamnett, A. & Vielstich, W.Electrochemistry, chap. 4, 191–194 (Wiley-VCH, Weinheim, 1998), 2 edn.
Keeler, J. Understanding NMR Spectroscopy, chap. 8, 1–24 (John Wiley and Sons, Chichester, U.K., 2010), 2 edn.
Zhang, M. & Reddy, R. Electrical field and current density distribution modeling of aluminum electrodeposition in ionic liquid electrolytes. ECS Trans. 1, 47–60 (2019).
Carr, H. Y. & Purcell, E. M. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev. 94, 630–638 (1954).
Meiboom, S. & Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 29, 688–691 (1958).
Bain, A. D. & Cramer, J. A. Optimal NMR measurements for slow exchange in two-site and three-site systems. J. Phys. Chem. 97, 2884–2887 (1993).
Newville, M. et al. lmfit/lmfit-py 0.9.14. Zenodo (2019).
Neese, F. Software update: The ORCA program system–version 5.0. Wiley Interdiscip. Rev.: Computational Mol. Sci. 12, e1606 (2022).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zetavalence and quadruple zeta valence quality for H to Rn: Design and assessmentof accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132 (2010).
Stoychev, G. L., Auer, A. A. & Neese, F. Automatic generation of auxiliary basis sets. J. Chem. Theory Comput. 13, 554–562 (2017).
Acknowledgements
We would like to thank Philipp Schleker for the scientific discussions on solution and catalyst chemistry and Simone Köcher for her advice and suggestions on chemical shift calculations. We also gratefully acknowledge financial support by the German Federal Ministry of Education and Research (BMBF) within the Kopernikus Project P2X: Flexible use of renewable resources—research, validation and implementation of ‘Power-to-X’ concepts, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—Cluster of Excellence 2186 “The Fuel Science Center". Computational resources from RWTH Aachen University under project rwth1253 are acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Sven Jovanovic performed the in operando experiments and contributed major parts to the data analysis and evaluation. Davis Thomas Daniel performed the DFT chemical shift calculations. Peter Jakes, Steffen Merz, Rüdiger-A. Eichel, and Josef Granwehr contributed to the interpretation and evaluation of experiments and simulations, and supervised the research project. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Communications Chemistry thanks Jian Zhi Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jovanovic, S., Jakes, P., Merz, S. et al. In operando NMR investigations of the aqueous electrolyte chemistry during electrolytic CO2 reduction. Commun Chem 6, 268 (2023). https://doi.org/10.1038/s42004-023-01065-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-01065-3
This article is cited by
-
The road to the electroreduction of CO2
Communications Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.