Abstract
Biomolecular coacervates are emerging models to understand biological systems and important building blocks for designer applications. DNA can be used to build up programmable coacervates, but often the processes and building blocks to make those are only available to specialists. Here, we report a simple approach for the formation of dynamic, multivalency-driven coacervates using long single-stranded DNA homopolymer in combination with a series of palindromic binders to serve as a synthetic coacervate droplet. We reveal details on how the length and sequence of the multivalent binders influence coacervate formation, how to introduce switching and autonomous behavior in reaction circuits, as well as how to engineer wetting, engulfment and fusion in multi-coacervate system. Our simple-to-use model DNA coacervates enhance the understanding of coacervate dynamics, fusion, phase transition mechanisms, and wetting behavior between coacervates, forming a solid foundation for the development of innovative synthetic and programmable coacervates for fundamental studies and applications.
Similar content being viewed by others
Introduction
Membraneless organelles (MLOs), also known as biomolecular condensates, form through liquid–liquid phase separation (LLPS) within cells and have been shown to play a crucial role in regulating biological functions1,2,3. Unlike traditional membrane-bound organelles, MLOs such as nucleoli, stress granules, and P-bodies function in the absence of a surrounding lipid bilayer. They are often formed by proteins or complexes of proteins and nucleic acids4,5,6. The unique properties of MLOs are predominantly driven by an array of weak interactions, including protein–protein, protein–RNA, electrostatic, and hydrophobic interactions, culminating in dynamic liquid properties7,8,9. These liquid characteristics influence the spatial organization, phase transition, and molecular interactions within cells, thereby regulating diverse cellular activities, including gene expression, cell adhesion, and cargo recruitment10,11,12.
Inspired by natural LLPS-driven organelles, the fabrication of bioinspired LLPS systems, consisting of DNA, RNA, and proteins, has gained much ground in order to better understand biological systems using defined model systems, as well as for generating functional materials relevant to the molecular systems engineering world13,14,15,16,17. Despite the progress, many open questions remain, for instance, regarding the interactions between coacervates of different natures and of coacervates with soft interfaces. Interesting progress has also been made with respect to the uptake of coacervates into liposomes or cells or, very recently, regarding LLPS in fibrillar environments18,19,20.
DNA is an appealing building block to build up coacervates due to the programmable nature of interactions21. Various strategies have been employed to construct DNA coacervates and droplets, including the use of hybridization-mediated assembly of DNA nanostars or temperature-induced phase segregation and trapping of DNA16,22,23,24,25. These all-DNA artificial cell models can exhibit liquid-like behavior, beneficial to study adhesion, fission, fusion, and wetting26,27,28,29,30,31,32. For instance, Takinoue and co-workers investigated the impact of DNA sequences on the fusion dynamics of liquid-like droplets at elevated temperatures (above 43 °C)28. However, many of the approaches require specialist knowledge of DNA assembly techniques and well-designed annealing protocols, whereas simple mix-and-use protocols at one temperature would be very desirable.
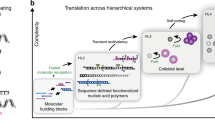
In this study, we report an easy-to-use platform for all-DNA coacervates utilizing single-stranded DNA (ssDNA) components and multivalency-driven LLPS at physiological temperature (Fig. 1). We show that a series of palindromic domains can be flexibly hybridized to long ssDNA homopolymers composed solely of adenine nucleotides (polyA) to trigger coacervate formation, and to study dynamics as a function of the type of palindromic binder. Through the integration of DNA or RNA strands, we establish switchable systems for the controlled assembly and disassembly of coacervates. This advancement enables the realization of a transient, autonomous coacervate system33. Furthermore, the selective exploitation of the distinctive dynamic properties conferred by various palindromic domains allows to engineer multiphase structures through tailored wetting mechanisms. We anticipate that the facile formation mechanism and the flexible design of different dynamic behaviors of these DNA coacervates will pave the way for the engineering of functional DNA-based coacervates, with great potential to be followed up by non-experts.
DNA coacervates are prepared via multivalency-driven liquid–liquid phase separation (LLPS) between a ssDNA polymer (polyA) and shorter self-complimentary binders (Bx). The introduction of DNA or RNA invaders triggers the formation of switchable and transient coacervates. Using two distinct coacervates with different palindromic domains allows controlling multiphase structures through engineering wetting.
Results
Formation of multivalency-driven coacervates
Our strategy for the fabrication of all-DNA coacervates employs a straightforward multivalency-driven LLPS approach based on the combination of a ssDNA homopolymer (polyA; A = adenine) and shorter binders (Bx) containing palindromic domains and a T30 (T = thymine) domain for hybridization to the polyA backbone (Fig. 2a). The DNA homopolymer A1500 was synthesized using terminal deoxynucleotidyl transferase (TdT)-catalyzed polymerization to feature 1500 repeating units34,35. Similar polyAs are, however, also available commercially. The binders, Bx, typically feature four components: (1) the complementary segment T30 ensuring hybridization to the polymer A1500 (melting temperature, Tm (A30/T30) = 62.8 °C at 10 mM Mg2+, 50 mM Na+, by IDT), (2) an intermediate spacer providing both flexibility and a toehold (S7), (3) the palindromic domain, Px, facilitating multivalent interactions, and (4) a dye label. These structures are hence composed of T30–S7–Px (or shorter Bx), whereby x signifies the length of the palindrome and thus the strength of the interaction. The strength of the palindrome also depends on the GC content. The table listing the Tm in Fig. 2b gives a rough correlation of the binder strength.
a Multivalency-driven coacervates by a long ssDNA (A1500) mixed with short binder ssDNA consisting of a complementary strand T30, a spacer S7 and a palindromic multivalency domain Px. b Sequences and melting temperatures (Tm) of the palindromic domains at 10 mM Mg2+. c Size distribution of coacervates in systems containing 0.06 µM A1500 and 1.5 µM binder at 37 °C after a 4 h reaction period with various palindromic domains. Error bars correspond to standard deviations of ca. 150 coacervates. d CLSM images of systems containing 0.06 µM A1500 and 1.5 µM binder at 37 °C after a 4 h reaction period with different palindromic domains. e Time-dependent CLSM images of A1500/B8-1. The fluorescent labels are on the Bx. The experiments were conducted at 37 °C with gentle rotation at 80 rpm. Scale bars: 10 μm.
We designed eight distinct binder strands (T30–S7–Px = Bx), each characterized by a unique sequence and varying length of the palindromic domain (Fig. 2b). Through this approach, we aimed to understand the influence of these two factors (GC content and palindromic length) on the formation and dynamics of spherical coacervates. Coacervate systems were typically assembled by mixing Bx with A1500 in a molar ratio of 25:1 at 1.5 µM T30–S7–Px and 0.06 µM A1500 at 37 °C in a buffer containing 10 mM Mg2+ and 50 mM K+ (pH = 7.9). Note that 0.06 µM A1500 is equivalent to 3 µM A30, and hence the Bx segments can occupy 50% of all A repeat units in A1500. Confocal laser scanning microscopy (CLSM) shows nicely spherical coacervates for five of the eight systems (Fig. 2c, d; Supplementary Data 1). The system featuring the shortest Px, comprised of merely four nucleotides (nt) and a low GC content (P4-1), presents no distinguishable structure formation. Systems with the longest Px, containing 10 or 12 nt (P10, P12), display excessive aggregation into non-spherical structures. This behavior roughly correlates with the Tm of the individual palindromes, but the multivalent strengthening needs to be considered as well.
The absence of coacervate formation in the A1500/P4-1 system (Tm,P4-1 = 0 °C) arises from the feeble multivalent binding due to the low GC content. Even though the other shorter Px systems (x = 4-2, 6, 8-1, 8-2, 8-3) have also calculated Tm < 28 °C, hence still significantly lower than the system temperature of 37 °C, they still lead to the formation of well-defined spherical coacervates due to efficient multivalency effects. This indicates a dynamic nature and binding/unbinding dynamics. In contrast, the aggregation in systems incorporating the longest Px strands (x = 10, 12) with Tm = 38.7 and 41.9 °C can be attributed to the excessively rigid multivalent interactions without significant internal dynamics for rearrangement into spherical structures. Hence, for the fabrication of spherical all-DNA coacervate via multivalency-driven LLPS, the optimum length of Px containing four GC bases lies in the approximate range of 4–8 nt in total (Fig. 2c). This balances phase separation and sufficient rebinding dynamics on a multivalency level, yielding the desired spherical structural output.
We monitored a mixture of A1500 and B8-1 at 37 °C for visual tracking of the coacervate formation. After 0.5 h, a population of small coacervates with an average diameter of 0.7 µm appears. These small coacervates rapidly coalesce, gradually increase in size, and transform into larger entities with an average diameter of ca. 2.8 µm within 1 h. In the following 30 h, these structures continue to progressively coalesce and evolve into well-defined coacervates with ca. 8.8 µm diameter (Fig. 2e, Supplementary Fig. 1). This coalescence and growth further highlight the dynamic behavior within the phase-separated coacervate state. Fluorescence recovery after photobleaching (FRAP) measurements reveal a partial, limited recovery within 1.5 h post bleaching for the three coacervates with 8 nt in the palindrome. At the same time, partial fusion events can be clearly observed (Supplementary Fig. 2; arrows therein highlight fusion events; Supplementary Fig. 3 shows the minor effect of palindromic domains with different lengths = 4, 6, 8 nt). Hence, these coacervates are sufficiently dynamic to allow for fusion. Most interestingly, the coacervates formed by B8-2 show the quickest recovery at the coacervate surface. Hence, despite the strong similarities in the different binders of the B8-y series, subtle differences in behavior can occur.
DNA- and RNA-triggered coacervate dynamics: switchable and transient systems
In the above investigation, we discussed the mechanism and conditions guiding the formation of our multivalency-driven coacervates. To achieve switchable and transient coacervate systems, we employed tools from DNA and RNA nanoscience to coacervates formed with the binder B8-1. For the establishment of switchable coacervates, we introduced a DNA invader consisting of a domain complementary to the S7-P8-1 segment of the binder, along with an additional dangling overhang acting as a toehold for subsequent reactions (Fig. 3a; all sequences in Table S1). The addition of this invader to already formed coacervates disengages the multivalent interactions via strand displacement of the Px/Px palindrome. CLSM images demonstrate the disappearance of the coacervates upon the addition of the DNA invader in less than 10 minutes (Fig. 3b). Furthermore, the introduction of an additional DNA anti-invader, which can hybridize with the DNA invader at its dangling overhang, triggers displacement of the invader and the re-engagement of the palindromic hybridization site followed by reassembly of the coacervate. This process enables simple isothermal switching of the DNA coacervates.
a and b Illustration and CLSM images of DNA-triggered switchable coacervate systems. 2.25 µM (1.5 eq.) of DNA invader was introduced to the coacervates formed from 0.06 µM A1500 and 1.5 µM (1.0 eq.) of B8-1, resulting in the disassembly of the coacervates. Subsequently, the addition of 3 µM (2.0 eq.) of DNA anti-invader caused the reformation of coacervates. c and d Illustration and CLSM images of RNA-triggered switching between coacervate and solution states. 4.5 µM (3 eq.) of RNA invader was introduced to the coacervates formed from 0.06 µM A1500 and 1.5 µM (1.0 eq.) of B8-1, resulting in the disassembly of the coacervates. Subsequently, the addition of 0.1 U µL−1 of RNase H triggered the assembly of coacervates. e and f Illustration and CLSM images of RNA-triggered switchable coacervate of solution-to-coacervate-to-solution. 15 µM (10 eq.) of RNA anti-invader was introduced into the complex comprising 0.06 µM A1500, 1.5 µM B8-1, and 2.25 µM DNA invader, leading to the assembly of the coacervates. Subsequently, the addition of 0.1 U µL−1 of RNase H caused the coacervates to disassemble into a homogeneous solution. A few tiny objects in the initial state may be due to the incomplete dissolution of the dye-bearing strand adsorbed in the microscopy chamber. g–k RNA-regulated transient coacervate disassembly with the introduction of RNA invader and RNase H: g Schematic illustration. h Time-dependent plot of the transient coacervates with tunable reassembly times by varying the concentration of RNA invaders. The green line indicates starting from a similar population of coacervates. Error bars are the standard deviation of ca. 20 droplet counts. i Controlled reassembly times via the concentration ratios of RNA invader/binder. Error bars correspond to standard deviations from duplicates. j Controlled reassembly times via the RNase H concentrations. Error bars correspond to standard deviations from duplicates. k Time-dependent CLSM images of transient coacervates with 15 µM (10 eq.) RNA invader and 0.1 U µL−1 RNase H. l and m RNA-regulated transient coacervate assembly with the introduction of RNA anti-invader and RNase H: l Schematic illustration. m Time-dependent CLSM images of transient coacervates with 45 µM (30 eq.) RNA anti-invader and 0.1 U µL−1 RNase H. The experiments were conducted at 37 °C with gentle rotation at 80 rpm. Scale bars: 10 μm.
This concept can be extended to an RNA invader capable of complementary interaction with the S7–P8-1 segment of the binder (Fig. 3c, d, Supplementary Fig. 4). When RNA invaders are introduced, the coacervates disassemble rapidly in less than 10 minutes. Coacervate reassembly occurs upon the addition of RNase H, which specifically degrades RNA hybridized with DNA. A toehold is no longer needed as for the DNA invader. The use of an enzyme allows, in principle, to fine-tune the kinetics of the re-engagement by simply changing its concentration. Furthermore, the switching process can be inverted by introducing an RNA anti-invader that removes a blocker strand from the Bx domain to trigger assembly. Subsequent addition of RNase H thereafter affords disassembly of the coacervate systems by degradation of the RNA anti-invader and subsequent reblocking of the Bx with the newly liberated original ssDNA block strand (Fig. 3e,f).
Building upon the RNA-triggered switchable coacervate systems, we asked whether autonomous and transient coacervate dissolution, as well as coacervate formation, would be possible by the addition of RNA trigger strands into RNase H-loaded systems36,37,38. Fig. 3g–k displays transient coacervate disassembly. Coacervates formed by A1500 and B8-1 were first generated and then combined with an excess of RNA invader (10 eq. of binder) at a low concentration of RNase H (0.1 U µL−1). Before introducing the RNA invader, CLSM images show the initially formed coacervates (Fig. 3k). The subsequent simultaneous addition of RNA invader and RNase H set off an autonomous and dynamic transformation between coacervate and solution states. Within 10 min, the coacervates disassemble into a fully homogeneous solution due to rapid hybridization of B8-1 and RNA invader, which blocks multivalent interactions. After 0.5 h, some minor coacervates are observed because of the gradual degradation of the RNA invader by the already present RNase H. As RNA degradation continues, the reformation of well-defined coacervates occurs (Fig. 3h, k; Supplementary Data 1). Importantly, the reassembly time (defined as the point where coacervates reappear in CLSM) of these transient coacervates can be adjusted within a range of 0.3–2.9 h by either augmenting the quantity of RNA invader or reducing the concentration of RNase H (Fig. 3i, j; Supplementary Figs. 5 and 6; Supplementary Data 1).
The opposite pathway, i.e., transient coacervates, can be accomplished by integrating the A1500/B8-1/DNA-invader complex with an excess of RNA anti-invader and a low concentration of RNase H (Fig. 3l). The RNA anti-invader quickly removes the DNA strand blocking the multivalent interaction, and thereby activated coacervation. By balancing the competition between anti-invader/invader hybridization and anti-invader degradation by RNase H and allowing the necessary time for coacervate formation, well-defined coacervate structures are observed after 1.5 h (Fig. 3m, Supplementary Fig. 7). After 3 h, these coacervates gradually disappear. Strikingly, hollow capsules appear, which indicates entrapment of the RNase H due to affinity to RNA (and potentially DNA) inside the coacervates and degradation from the inside. The relatively high stability of the vacuoles without diffusion to the surface likely originates from the viscoelastic character of the coacervates with limited dynamics (FRAP in Supplementary Fig. 2). Such vacuole formation has been observed earlier for enzymatic degradation of DNA nanostar condensates when adding a restriction enzyme from the outside and was associated with the dynamics of the condensate and enzyme migration dynamics39. Our mechanism is, however, different as the restriction enzyme is present from the start and entrapped and does not need to diffuse into the condensate. Both approaches highlight the development of RNA-mediated autonomous transient coacervate systems that function by simultaneously utilizing RNA strands and the endoribonuclease. These systems will be used below for slow activation of binary coacervate systems.
Engineering wetting between coacervates
After understanding how the combination of A1500 and Bx can lead to the formation of dynamic and switchable coacervates, we hypothesized that these simple-to-prepare coacervates can be used to engineer coacervate mixtures and interactions using various binders. To this end, we prepared three distinct coacervates: C1 in magenta, C2 in green, and C3 in cyan, using different binders B8-1, B8-2, and B8-3, respectively, wherein each binder is associated with a specific and selectively binding palindrome sequence (P8-1, P8-2, and P8-3, as shown in Figs. 2b, c and 4a). After the initial formation of the individual coacervates (time = 1 h; 37 °C), we combined two of them to understand whether they remain segregated or potentially fuse despite being built by different palindromic binders.
The coacervates were prepared using a mixture containing a final concentration of 0.06 µM of A1500 and 1.5 µM of the respective Bx. a Illustration of three distinct coacervate structures (C1 in magenta, C2 in green, and C3 in cyan) synthesized from different binders (Atto647N–B8-1, Atto488–B8-2, and Atto565–B8-3) with various palindromic sequences (P8-1, P8-2, and P8-3, as showed in Fig. 2b, c). The experiments were performed at 37 °C with gentle rotation at 80 rpm unless otherwise specified. b Wetting behavior between distinct C1 and C2, leading to the formation of mixed coacervates. c Wetting behavior between C1 and C3, resulting in the formation of core–shell structures. d Wetting behavior between C2 and C3, contributing to the formation of core–shell structures. The experiments were carried out at 37 °C without rotation to minimize collisions. e Behavior during RNase H-initiated, slow coacervation of C1 in a dispersion of preformed C2. 0.1 U µL−1 RNase H and preformed C2 were introduced into the complex comprising 0.06 µM A1500, 1.5 µM of B8-1, and 2.25 µM RNA invader. f Behavior during RNase H-initiated, slow coacervation of C1 to preformed C3. 0.1 U µL−1 RNase H and preformed C3 were introduced into the complex comprising 0.06 µM A1500, 1.5 µM of B8-1, and 2.25 µM RNA invader. The experiments were conducted at 37 °C with gentle rotation at 80 rpm. Scale bars: 10 μm.
Following an 11-day incubation period at 37 °C, a rich diversity of morphologies emerges. In general, the process follows initial contact at early times, subsequent engulfment, formation of a preferred wetting layer, and ultimately complete mixing (Fig. 4). The harmonization process occurs on different time scales for the different coacervate mixtures and is not complete for mixtures of C1 (magenta)/C3 (cyan) and of C2 (green)/C3 (cyan), for which distinct core–shell morphologies persist with slow homogenization even after 11 days (Fig. 4c, d), These observations are in line with the diminished dynamics of C3 relative to C1 and C2 (Supplementary Fig. 2). The tendency to form the wetting layer on the surface follows C1 < C2 < C3. It leaves us to speculate that the small differences in palindrome binder—despite being very similar in structure and thermodynamics—leads to slightly different surface tensions. It is interesting to note that the most hydrophilic dye (Atto488) located on C2 (green) does not form the most effective surface wetting layer (Fig. 4d). Concerning the gradual homogenization, control experiments by gel electrophoresis point to a gradual strand displacement within simplified model system of A37/B8-1 and B8-2 (Supplementary Fig. 8), wherein the initially bound B8-1 strand is undergoing slow exchange with B8-2. This process may aid in compatibilization. Note that NUPACK simulations do not indicate cross-talk/off-target binding between the different B8−x binders down to a temperature of 5 °C.
To further investigate the emergence of wetting phenomena, we employed the autonomous formation of one coacervate system in the presence of an already formed different coacervate. This connects to Fig. 3c, where RNase H-mediated digestion of RNA was used to trigger coacervation. When using slow RNase H-mediated formation of C1 in the presence of already preformed C2 (Fig. 4e), the data shows homogeneous nucleation of distinct magenta C1 structures in the presence of preformed C2. Heterogeneous nucleation is absent. Over time, these discrete coacervates form a unified coacervate. When slowly activating C1 to preformed C3 (Fig. 4f), again homogeneous nucleation takes place followed by the formation of core-shell structures. Hence the morphological development is independent of the nucleation pathway and the temporal orchestration. No matter whether preformed coacervates are mixed or whether one is grown slowly, similar fully mixed or core/shell structures appear. This points to the robustness of the process. Overall, this approach shows how to control diverse morphologies in the presence of two distinct coacervate systems, thereby not only achieving wetting control but also mimicking the formation processes and wetting behaviors of the biological organelles within cells29,40,41,42.
Obviously, the wetting process between two preformed coacervates takes more than one week because of the slow mobility of the coacervates, as well as the very slow possible exchange of binder segments. To gain a better understanding of the wetting behavior and accelerate this process, we employed a so-called C31 system together with the C1 coacervates (Fig. 5). The C31 coacervate was engineered to contain mixtures of its original B8-3 binder and either 25 or 50 mol% of the B8-1 binder (Fig. 5a). The latter serves to strengthen and accelerate interactions between the C31 and the C1.
Coacervates C1 (or C3) were formed using a mixture containing a final concentration of 0.06 µM of A1500 and 1.5 µM of either B8-1 or B8-3. Coacervates C31 were generated from 0.06 µM A1500 and a mixture totaling 1.5 µM of B8-1 and B8-3. a Illustration of three distinct coacervate structures (C1 in magenta, C3 in cyan, and C31 in dark cyan) synthesized using different binders (B8-1, B8-3, and a mixture of B8-3 and B8-1). The experiments were performed at 37 °C with gentle rotation at 80 rpm unless otherwise specified. b Wetting behavior upon the combination of C1 and C3. c Accelerated wetting behavior upon the combination of C1 and C31 by the introduction of B8-1 in the C31 system at a ratio of 75%:25% B8-3:B8-1 (1.125 µM of B8-3 and 0.375 µM of B8-1). d Accelerated wetting behavior upon the combination of C1 and C31 by introducing B8-1 in the C31 system at a ratio of 50%:50% B8-3:B8-1 (0.75 µM of B8-3 and 0.75 µM of B8-1). The experiments were conducted at 37 °C without rotation to minimize collisions. e Suggested mechanisms of coacervate fusion for C1 and C3. The molecular exchange between different-type coacervates occurs through either detachment of the binders and their diffusion or strand displacement. Scale bars: 10 μm.
Upon mixing C1 and C31 (Fig. 5c, d), core–shell structures visually identical to the C1/C3 system are formed (Figs. 4c and 5b), but with different formation times, based on the ratio of B8-3 to B8-1. C31 with a B8-3:B8-1 ratio of 75%:25% leads to the observation of Janus-like structures within 1 day. Following this, multicompartment structures occur after 3 days, and the final core–shell structures emerge within 7 days (Fig. 5c). Furthermore, changing the B8-3:B8-1 ratio to 50%:50% significantly enhances the wetting behavior, resulting in the emergence of the final core–shell structures within 3 days, instead of 7 days (Fig. 5d). This phenomenon indicates that the wetting speed can be effectively modulated by tuning the multivalent interactions of the binders between two coacervates.
The wetting behavior of C1 and C31 helps to understand the interaction in a mixture of pure, distinct C1 and C3. In the mixture of C1 and C3, as mentioned above, the unbound free A repeat units on A1500 within coacervates present opportunities for strand exchange between B8-1 in the C1 coacervate and B8-3 in the C3 coacervate (Fig. 5e; Supplementary Fig. 8). This strand displacement process leads to an increased presence of B8-3 in C1 as well as of B8-1 in C3 that enables multivalent interactions between the original C1 and C3 via wetting and engulfment. This strand displacement behavior can occur when the coacervates come into contact. Subsequently, the strand exchange enhances the emergence of complex structures through wetting, fusion, and engulfment. The process is sketched in Fig. 5e. We hypothesize that strand exchange may also occur very slowly, even when the coacervates are at a distance from each other through complete detachment of the binders and their diffusion (equilibrium dynamics of dsDNA). It is reasonable to assume that elongation of the Tx segment in the binders would further limit the molecular exchange and thus slow down or even prevent fusion.
Conclusions
In summary, we introduced a simple and versatile platform for programmable DNA coacervates. In contrast to many other DNA coacervate systems, this model system does not require much knowledge on the design of DNA nanostars, complicated synthesis or well-designed annealing protocols, but operates on a simple mix-and-use principle. Furthermore, due to the presence of a polymeric component (polyA), a different viscoelastic regime can be generated within the coacervates as compared to nanostar-based droplets, because polymer entanglements can be present for long polyA, as well as higher multivalency effect. FRAP measurements indicate a slow and partial recovery in the coacervates, contrasting with the rapid and almost complete recovery reported in nanostar-based droplets by previous studies22,43,44,45. Even though we synthesized the polyA building block in-house, it is commercially available, and preliminary experiments show that the commercial polyA also undergoes coacervation (Supplementary Fig. 9).
Using established strand displacement switching principles of DNA nanoscience, we demonstrated simple switching of our coacervates. The assembly/disassembly switching can also be achieved using RNA/RNase H switches that allow fine-tuning of the kinetics and the design of autonomous systems, where recruitment of the enzymes into the coacervate droplet was observed. The liquid-like properties and small differences in the palindrome binder of these coacervates paved the way for engineering wetting, clustering, engulfment, and merging of the distinct coacervate droplets. The interaction of these coacervates can be simply engineered by co-hybridization with other linkers, which should allow for sequential mixing processes in the future.
The facile tunability and the facile options for functionalization should enable fundamental studies into coacervate dynamics and structures, as well as open doors for applications in delivery, synthetic biology, and material science.
Methods
Synthesis of ssDNA polymer A1500 by TdT polymerization
TdT polymerization is a widely employed method for synthesizing ssDNA products through controlled nucleotide polymerization. To synthesize A1500, a mixture of 0.2 µM primer, 300 µM dATP, 1x TdT buffer, and 1 U µL−1 TdT enzyme (Supplementary Materials) was prepared in nuclease-free water, resulting in a final volume of 150 µL. The reaction was conducted at 37 °C for 2 h and then quenched by adding 20 µL of 200 mM EDTA solution. The obtained polymer was purified using 10 kDa amicon ultra centrifugal filters and washed three times with TE buffer. The concentration of the polymer was determined using UV–Vis spectroscopy (Supplementary Characterization Methods and Instrument).
Palindromic effect on coacervate formation
To evaluate the impact of the palindromic length and GC content on the multivalency-driven coacervation, eight different binder strands with varying palindromic lengths and sequences were introduced (Supplementary Table 1). The samples were prepared by dissolving 0.03 g L−1 (~0.06 µM) A1500 and 1.5 µM binder in rCutSmart buffer containing 10 mM Mg2+ and 50 mM K+ (pH = 7.9), resulting in a final volume of 20 µL. The solutions were incubated for 4 h at 37 °C with gentle rotation at 80 rpm. The resulting structures were visualized and recorded using CLSM. The experiments were conducted using a 384-well microtiter plate with a 0.2 mm glass bottom.
Time-dependent coacervate formation
A 20 µL solution was prepared containing 0.06 µM of A1500 and 1.5 µM of B8-1 in rCutSmart buffer. This solution was incubated for 30 h at 37 °C with a rotation of 80 rpm. The coacervate formation process was recorded using CLSM.
DNA-triggered switchable coacervate
To realize a transformation sequence from coacervate to solution and back to coacervate (coacervate-to-solution-to-coacervate), coacervates containing B8-1 were prepared with a final concentration of 0.06 µM A1500 and 1.5 µM (1.0 eq.) of B8-1 in rCutSmart buffer. This mixture was incubated for 12 h at 37 °C with gentle rotation at 80 rpm. Subsequently, 2.25 µM (1.5 eq.) of DNA invader was introduced and allowed to incubate for 0.5 h, resulting in the disassembly of the coacervates. Following this, 3 µM (2.0 eq.) of DNA anti-invader was added and incubated for an additional 16 h, leading to the reformation of coacervates. Images were recorded during this process using CLSM.
RNA-triggered switchable coacervate
In the switchable system between coacervate and solution states, coacervates containing B8-1 were prepared with a final concentration of 0.06 µM A1500 and 1.5 µM (1.0 eq.) of B8-1 in rCutSmart buffer. This mixture was incubated for 1 h at 37 °C with gentle rotation at 80 rpm. Subsequently, RNA invader (4.5 µM, 3 eq.) was introduced and allowed to incubate for 10 min until the coacervates disappeared. Following this, 0.1 U µL−1 RNase H was added and incubated overnight.
For the transformation sequence of solution-to-coacervate-to-solution, a mixture containing 0.06 µM A1500, 1.5 µM (1.0 eq.) B8-1, and 2.25 µM (1.5 eq.) DNA invader was prepared in rCutSmart buffer (complex of A1500/B8-1/DNA invader). The solution was rapidly heated to 80 °C at a rate of 3 °C s−1 and then gradually cooled to 20 °C at a rate of 0.01 °C s−1. Subsequently, RNA anti-invader (15 µM, 10.0 eq.) was added to the annealed solution and incubated for 1 h to form coacervates. Following this, 0.1 U µL−1 RNase H was introduced and incubated for 2 h, resulting in the coacervates disassembling into a homogeneous solution.
RNA-regulated transient coacervate
For the RNA-regulated transient coacervate disassembly system, the coacervates were first prepared with final concentrations of 0.06 µM of A1500 and 1.5 µM (1.0 eq.) of B8-1 in rCutSmart buffer. The mixture was incubated for 2 h at 37 °C with gentle rotation at 80 rpm. Following this, varying amounts of RNA invader (15, 45, 75 µM) and 0.1 U µL−1 RNase H were added to the solution. The mixture was then incubated at 37 °C with gentle rotation at 80 rpm. Images were recorded during this process using CLSM, and the tunable reassembly time was monitored. Additionally, to further explore the effect of RNase H concentration on the reassembly time, RNA invader (15 µM, 10.0 eq.) and different RNase H concentrations (0.03, 0.05, 0.1 U µL−1) were introduced. The duration of reassembly is measured from the moment the preformed coacervates disassemble to the commencement of coacervate reformation.
To achieve the opposite pathway (transient coacervate assembly), RNA anti-invader and RNase H were introduced. The complex of A1500/B8-1/DNA invader was prepared with a final concentration of 0.06 µM A1500, 1.5 µM (1.0 eq.) B8-1, and 2.25 µM (1.5 eq.) DNA invader in rCutSmart buffer. The solution was rapidly heated to 80 °C at a rate of 3 °C s−1 and then gradually cooled to 20 °C at a rate of 0.01 °C s−1. Then different concentrations of RNA anti-invader (15, 45 µM) and RNase H (0.1 U µL−1) were added to the solution. The mixture was incubated at 37 °C with gentle rotation at 80 rpm, and images were recorded during this process using CLSM.
Engineering wetting between coacervates
Three distinct coacervate structures, denoted as C1, C2, and C3, were prepared using different binders (B8-1, B8-2, and B8-3). A mixture with a final concentration of 0.06 µM A1500 and 1.5 µM of the respective Bx was dissolved in rCutSmart buffer and incubated for 1 h at 37 °C with gentle rotation at 80 rpm. Subsequently, two of these resulting coacervates were mixed and incubated for 11 days at 37 °C without shaking (shaking was avoided in this case to limit collisions). The morphological changes during this period were captured and recorded using CLSM.
Engineering wetting under nucleation process by activating C1 to preformed C2 (or C3)
To investigate the structures that can emerge through the wetting behavior during the nucleation process, we introduced a system where B8-1 is blocked by an RNA invader. Coacervates of C2 and C3 were prepared as described previously. A mixture consisting of A1500 (0.06 µM), B8-1 (1.5 µM, 1.0 eq.), and RNA invader (2.25 µM, 1.5 eq.) was prepared in rCutSmart buffer, forming the complex of A1500/B8-1/RNA invader. The solution was rapidly heated to 80 °C at a rate of 3 °C s−1 and then gradually cooled to 20 °C at a rate of 0.01 °C s−1. Subsequently, 0.1 U µL−1 RNase H and preformed C2 (or C3) were introduced into the solution and incubated for 72 h at 37 °C with gentle rotation at 80 rpm. The morphological changes occurring during this period were monitored and recorded using CLSM.
Accelerated wetting between two coacervates
To facilitate the wetting process, coacervate C31, incorporating different ratios of B8-1 and B8-3 (B8-3:B8-1 = 75%:25%, 50%:50%), was employed. In the system with 75%:25% B8-3:B8-1, a mixture comprising 1.125 µM of B8-3 and 0.375 µM of B8-1 was combined with 0.06 µM A1500 in rCutSmart buffer. This solution was then incubated for 1 h at 37 °C with gentle rotation at 80 rpm to form C31. C1 was prepared as described previously. Subsequently, C31 solution was mixed with the C1 solution and incubated for 7 days at 37 °C without shaking. Morphological changes during this period were observed and recorded using CLSM.
Strand exchange reaction between A37/B8-1 and B8-2
A mixture was prepared by mixing 2 µM A37 and 10 µM B8-1 in rCutSmart buffer, resulting in a final volume of 20 µL. This solution was rapidly heated to 80 °C at a rate of 3 °C s−1 and then slowly cooled to 20 °C at a rate of 0.01 °C s−1. Then 10 µM B8-2 was added to the solution, and the mixture was incubated for 24 h at 37 °C. The samples were analyzed through agarose gel electrophoresis using a 4 wt% agarose gel.
Coacervate formation using commercial polyA
A 20 µL solution containing 0.06 µM of polyA and 2.5 µM of B8-1 in rCutSmart buffer was prepared. This solution was incubated at 37 °C with a rotation of 80 rpm, and images were recorded during this process using CLSM.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all relevant data supporting the findings of this study are available within the paper and its Supplementary Information file. The source data for Figs. 2c and 3h-j are included in Supplementary Data 1. Additional data related to this study are available from the corresponding author upon reasonable request.
References
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Garabedian, M. V. et al. Designer membraneless organelles sequester native factors for control of cell behavior. Nat. Chem. Biol. 17, 998–1007 (2021).
Gomes, E. & Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 294, 7115–7127 (2019).
Hernandez-Verdun, D. Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2, 189–194 (2011).
Mao, Y. S., Zhang, B. & Spector, D. L. Biogenesis and function of nuclear bodies. Trends Genet. 27, 295–306 (2011).
Decker, C. J. & Parker, R. P-Bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 4, a012286 (2012).
Kaur, T. et al. Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies. Nat. Commun. 12, 872 (2021).
Liu, W., Samanta, A., Deng, J., Akintayo, C. O. & Walther, A. Mechanistic insights into the phase separation behavior and pathway‐directed information exchange in all‐DNA droplets. Angew. Chem. Int. Ed. 61, e202208951 (2022).
Milovanovic, D., Wu, Y., Bian, X. & Camilli, P. D. A liquid phase of synapsin and lipid vesicles. Science 361, 604–607 (2018).
Hirose, T., Ninomiya, K., Nakagawa, S. & Yamazaki, T. A guide to membraneless organelles and their various roles in gene regulation. Nat. Rev. Mol. Cell Biol. 24, 288–304 (2023).
Beutel, O., Maraspini, R., Pombo-García, K., Martin-Lemaitre, C. & Honigmann, A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179, 923–936 (2019).
Schuster, B. S. et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 9, 2985 (2018).
Yewdall, N. A., André, A. A. M., Lu, T. & Spruijt, E. Coacervates as models of membraneless organelles. Curr. Opin. Colloid Interface Sci. 52, 101416 (2021).
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid–liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Liu, W., Lupfer, C., Samanta, A., Sarkar, A. & Walther, A. Switchable hydrophobic pockets in DNA protocells enhance chemical conversion. J. Am. Chem. Soc. 145, 7090–7094 (2023).
Leathers, A. et al. Reaction-diffusion patterning of DNA-based artificial cells. J. Am. Chem. Soc. 144, 17468–17476 (2022).
Cook, A. B., Novosedlik, S. & van Hest, J. C. M. Complex coacervate materials as artificial cells. Acc. Mater. Res. 4, 287–298 (2023).
Lu, T. et al. Endocytosis of coacervates into liposomes. J. Am. Chem. Soc. 144, 13451–13455 (2022).
te Brinke, E. et al. Dissipative adaptation in driven self-assembly leading to self-dividing fibrils. Nat. Nanotechnol. 13, 849–855 (2018).
Spruijt, E. Open questions on liquid-liquid phase separation. Commun. Chem. 6, 23 (2023).
Wang, J., Li, Z. & Willner, I. Dynamic reconfigurable DNA nanostructures, networks and materials. Angew. Chem. Int. Ed. 62, e202215332 (2023).
Sato, Y., Sakamoto, T. & Takinoue, M. Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci. Adv. 6, eaba3471 (2020).
Merindol, R., Loescher, S., Samanta, A. & Walther, A. Pathway-controlled formation of mesostructured all-DNA colloids and superstructures. Nat. Nanotechnol. 13, 730–738 (2018).
Samanta, A., Hörner, M., Liu, W., Weber, W. & Walther, A. Signal-processing and adaptive prototissue formation in metabolic DNA protocells. Nat. Commun. 13, 3968 (2022).
Samanta, A., Sabatino, V., Ward, T. R. & Walther, A. Functional and morphological adaptation in DNA protocells via signal processing prompted by artificial metalloenzymes. Nat. Nanotechnol. 15, 914–921 (2020).
Jeon, B.-j, Nguyen, D. T. & Saleh, O. A. Sequence-controlled adhesion and microemulsification in a two-phase system of DNA liquid droplets. J. Phys. Chem. B 124, 8888–8895 (2020).
Deng, J. & Walther, A. Programmable and chemically fueled DNA coacervates by transient liquid–liquid phase separation. Chem 6, 3329–3343 (2020).
Sato, Y. & Takinoue, M. Sequence-dependent fusion dynamics and physical properties of DNA droplets. Nanoscale Adv. 5, 1919–1925 (2023).
Mangiarotti, A., Chen, N., Zhao, Z., Lipowsky, R. & Dimova, R. Wetting and complex remodeling of membranes by biomolecular condensates. Nat. Commun. 14, 2809 (2023).
Deng, J. & Walther, A. ATP-powered molecular recognition to engineer transient multivalency and self-sorting 4D hierarchical systems. Nat. Commun. 11, 3658 (2020).
Rubio-Sánchez, R., Mognetti, B. M., Cicuta, P. & Di Michele, L. DNA-origami line-actants control domain organization and fission in synthetic membranes. J. Am. Chem. Soc. 145, 11265–11275 (2023).
Li, Z. et al. Dynamic fusion of nucleic acid functionalized nano-/micro-cell-like containments: from basic concepts to applications. ACS Nano 17, 15308–15327 (2023).
Heinen, L. & Walther, A. Celebrating soft matter’s 10th anniversary: approaches to program the time domain of self-assemblies. Soft Matter 11, 7857–7866 (2015).
Tang, L., Navarro, L. A. Jr, Chilkoti, A. & Zauscher, S. High‐molecular‐weight polynucleotides by transferase‐catalyzed living chain‐growth polycondensation. Angew. Chem. Int. Ed. 56, 6778–6782 (2017).
Fowler, J. D. & Suo, Z. Biochemical, structural, and physiological characterization of terminal deoxynucleotidyl transferase. Chem. Rev. 106, 2092–2110 (2006).
Green, L. N. et al. Autonomous dynamic control of DNA nanostructure self-assembly. Nat. Chem. 11, 510–520 (2019).
Sun, M., Deng, J. & Walther, A. Communication and cross-regulation between chemically fueled sender and receiver reaction networks. Angew. Chem. Int. Ed. 62, e202214499 (2023).
Farag, N., Dordevic, M., Del Grosso, E. & Ricci, F. Dynamic and reversible decoration of DNA-based scaffolds. Adv. Mater. 35, 2211274 (2023).
Saleh, O. A., Jeon, B. J. & Liedl, T. Enzymatic degradation of liquid droplets of DNA is modulated near the phase boundary. PNAS 117, 16160–16166 (2020).
Yoo, B. Y. & Chrispeels, M. J. The origin of protein bodies in developing soybean cotyledons: a proposal. Protoplasma 103, 201–204 (1980).
Ambroggio, E. E., Costa Navarro, G. S., Pérez Socas, L. B., Bagatolli, L. A. & Gamarnik, A. V. Dengue and Zika virus capsid proteins bind to membranes and self-assemble into liquid droplets with nucleic acids. J. Biol. Chem. 297, 101059 (2021).
Snead, W. T. et al. Membrane surfaces regulate assembly of ribonucleoprotein condensates. Nat. Cell Biol. 24, 461–470 (2022).
Agarwal, S., Osmanovic, D., Dizani, M., Klocke, M. A. & Franco, E. Dynamic control of DNA condensation. Nat. Commun. 15, 1915 (2024).
Maruyama, T., Gong, J. & Takinoue, M. Temporally controlled multistep division of DNA droplets for dynamic artificial cells. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2024-z67br (2024).
Skipper, K. & Wickham, S. Core–shell coacervates formed from DNA nanostars. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2024-07pvd (2024).
Acknowledgements
This work was supported by the DFG in WA 3084/19-1. W. L. acknowledges the support by the Chinese Scholarship Council (CSC).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
W.L. and A.W. conceived the project, designed the experiments, analyzed the data, and wrote the manuscript. W.L. performed the experiments and collected the data. J.D. contributed to the project design and data analysis. S. Song conducted experiments for synthesizing commercial polyA-based coacervates and supported the interpretation of the results. S. Sethi assisted with the discussions of the results. A.W. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, W., Deng, J., Song, S. et al. A facile DNA coacervate platform for engineering wetting, engulfment, fusion and transient behavior. Commun Chem 7, 100 (2024). https://doi.org/10.1038/s42004-024-01185-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-024-01185-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.