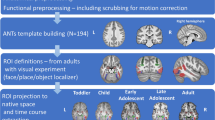
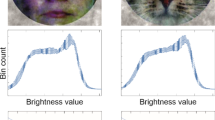
Abstract
Faces are thought to have a privileged status for processing relative to other visual images. Humans use faces to identify people, learn language, and to communicate and understand intentions, meaning and emotions. An enduring debate within the fields of developmental psychology and cognitive neuroscience is whether human face processing is specialized owing to domain-specific neural circuitry driven primarily by evolutionary mechanisms or whether it emerges from a domain-general architecture through experience. In this Perspective, we argue for an experience-based account based on associative and non-associative learning and supported by general neurobiological mechanisms. We posit that face-processing specialization emerges from activity-dependent, self-organizing processes where neuronal connectivity is shaped by the environment and constrained by intrinsic yet malleable neural architecture. This ‘domain-relevant’ framework for face processing reflects a dynamic interaction between the developing brain and the environmental input.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baillargeon, R. & Carey, S. in Early Childhood Development and Later Outcome (ed. Pauen, S. M.) 33–65 (Cambridge Univ. Press, 2012).
Carey, S. Précis of the origin of concepts. Behav. Brain Sci. 34, 113–124 (2011).
Spelke, E. S. & Kinzler, K. D. Core knowledge. Dev. Sci. 10, 89–96 (2007).
van der Lely, H. K. J. Domain-specific cognitive systems: insight from Grammatical-SLI. Trends Cogn. Sci. 9, 53–59 (2005).
Coltheart, M. Modularity and cognition. Trends Cogn. Sci. 3, 115–120 (1999).
Madole, K. L. & Oakes, L. M. Making sense of infant categorization: stable processes and changing representations. Dev. Rev. 19, 263–296 (1999).
Quinn, P. C. & Eimas, P. D. A reexamination of the perceptual-to-conceptual shift in mental representations. Rev. Gen. Psychol. 1, 271–287 (1997).
Rakison, D. H. & Lupyan, G. Developing object concepts in infancy: an associative learning perspective. VIII. General discussion. Monogr. Soc. Res. Child Dev. 73, 85–100 (2008).
Rogers, T. T. & McClelland, J. L. Semantic Cognition: A Parallel Distributed Processing Approach (MIT Press, 2004).
Smith, L. B., Jones, S. S. & Landau, B. Naming in young children: a dumb attentional mechanism? Cognition 60, 143–171 (1996).
Bates, J. E., Pettit, G. S., Dodge, K. A. & Ridge, B. Interaction of temperamental resistance to control and restrictive parenting in the development of externalizing behavior. Dev. Psychol. 34, 982–995 (1998).
Karmiloff-Smith, A. An alternative to domain-general or domain-specific frameworks for theorizing about human evolution and ontogenesis. AIMS Neurosci. 2, 91–104 (2015).
Farah, M. J., Rabinowitz, C., Quinn, G. E. & Liu, G. T. Early commitment of neural substrates for face recognition. Cogn. Neuropsychol. 17, 117–123 (2000).
McKone, E., Crookes, K., Jeffery, L. & Dilks, D. D. A critical review of the development of face recognition: experience is less important than previously believed. Cogn. Neuropsychol. 29, 174–212 (2012).
Sugita, Y. Innate face processing. Curr. Opin. Neurobiol. 19, 39–44 (2009).
Hildebrandt, A., Wilhelm, O., Schmiedek, F., Herzmann, G. & Sommer, W. On the specificity of face cognition compared with general cognitive functioning across adult age. Psychol. Aging 26, 701–715 (2011).
Tanaka, J. W. & Farah, M. J. Parts and wholes in face recognition. Q. J. Exp. Psychol. A 46, 225–245 (1993).
Yin, R. K. Looking at upside-down faces. J. Exp. Psychol. 81, 141–145 (1969).
Young, M. P. Objective analysis of the topological organization of the primate cortical visual system. Nature 358, 152–155 (1992).
Barton, J. J. Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage. J. Neuropsychol. 2, 197–225 (2008).
Takahashi, N. et al. Prosopagnosia: a clinical and anatomical study of four patients. Cortex 31, 317–329 (1995).
Polk, T. A., Park, J., Smith, M. R. & Park, D. C. Nature versus nurture in ventral visual cortex: a functional magnetic resonance imaging study of twins. J. Neurosci. 27, 13921–13925 (2007).
Wilmer, J. B. et al. Human face recognition ability is specific and highly heritable. Proc. Natl Acad. Sci. USA 107, 5238–5241 (2010).
Zhu, Q. et al. Heritability of the specific cognitive ability of face perception. Curr. Biol. 20, 137–142 (2010).
Quinones Sanchez, J. F., Liu, X., Zhou, C. & Hildebrandt, A. Nature and nurture shape structural connectivity in the face processing brain network. Neuroimage 229, 117736 (2021).
Kanwisher, N. Domain specificity in face perception. Nat. Neurosci. 3, 759–763 (2000).
Yovel, G. & Kanwisher, N. The neural basis of the behavioral face-inversion effect. Curr. Biol. 15, 2256–2262 (2005).
Deen, B. et al. Organization of high-level visual cortex in human infants. Nat. Commun. 8, 13995 (2017).
Kosakowski, H. L. et al. Selective responses to faces, scenes, and bodies in the ventral visual pathway of infants. Curr. Biol. 32, 265–274.e5 (2022).
Park, J., Newman, L. I. & Polk, T. A. Face processing: the interplay of nature and nurture. Neuroscientist 15, 445–449 (2009).
Morton, J. & Johnson, M. H. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev. 98, 164–181 (1991).
Gauthier, I. & Nelson, C. A. The development of face expertise. Curr. Opin. Neurobiol. 11, 219–224 (2001).
Nelson, C. A. in The Development of Face Processing in Infancy and Early Childhood: Current Perspectives (eds Pascalis, O. & Slater, A.) 79–97 (Nova Science, 2003).
Scott, L. S., Pascalis, O. & Nelson, C. A. A domain-general theory of the development of perceptual discrimination. Curr. Dir. Psychol. Sci. 16, 197–201 (2007).
Simion, F. & Giorgio, E. D. Face perception and processing in early infancy: inborn predispositions and developmental changes. Front. Psychol. 6, 969 (2015).
Hadley, H., Rost, G., Fava, E. & Scott, L. A mechanistic approach to cross-domain perceptual narrowing in the first year of life. Brain Sci. 4, 613–634 (2014).
Livingstone, M. S. et al. Development of the macaque face-patch system. Nat. Commun. 8, 14897 (2017).
Golarai, G., Ghahremani, D. G., Eberhardt, J. L. & Gabrieli, J. D. E. Distinct representations of configural and part information across multiple face-selective regions of the human brain. Front. Psychol. 6, 1710 (2015).
Scherf, K. S., Behrmann, M., Humphreys, K. & Luna, B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci. 10, F15–F30 (2007).
Arcaro, M. J., Schade, P. F., Vincent, J. L., Ponce, C. R. & Livingstone, M. S. Seeing faces is necessary for face-domain formation. Nat. Neurosci. 20, 1404–1412 (2017).
Smith, L. B., Colunga, E. & Yoshida, H. Knowledge as process: contextually-cued attention and early word learning. Cogn. Sci. 34, 1287–1314 (2010).
Markant, J. & Scott, L. S. Attention and perceptual learning interact in the development of the other-race effect. Curr. Dir. Psychol. Sci. 27, 163–169 (2018).
Scherf, K. S. & Scott, L. S. Connecting developmental trajectories: biases in face processing from infancy to adulthood. Dev. Psychobiol. 54, 643–663 (2012).
Jayaraman, S. & Smith, L. B. in The Cambridge Handbook of Infant Development: Brain, Behavior, and Cultural Context (eds Lockman, J. J. & Tamis-LeMonda, C. S.) 553–579 (Cambridge Univ. Press, 2020).
Bates, E. et al. in A Companion to Cognitive Science Ch. 46 (eds Bechtel, W. & Graham, G.) 590–601 (Blackwell, 1998).
Karmiloff-Smith, A. An alternative to domain-general or domain-specific frameworks for theorizing about human evolution and ontogenesis. AIMS Neurosci. 2, 91–104 (2015).
Farroni, T. et al. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. Proc. Natl Acad. Sci. USA 102, 17245–17250 (2005).
Jayaraman, S., Fausey, C. M. & Smith, L. B. The faces in infant-perspective scenes change over the first year of life. PLoS ONE 10, e0123780 (2015).
Jayaraman, S., Fausey, C. M. & Smith, L. B. Why are faces denser in the visual experiences of younger than older infants? Dev. Psychol. 53, 38–49 (2017).
Flanagan, J. G. Neural map specification by gradients. Curr. Opin. Neurobiol. 16, 59–66 (2006).
O’Leary, D. D. M., Yates, P. A. & McLaughlin, T. Molecular development of sensory maps. Cell 96, 255–269 (1999).
Rakic, P. Prenatal development of the visual system in rhesus monkey. Phil. Trans. R. Soc. Lond. B 278, 245–260 (1977).
Van Essen, D. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318 (1997).
Greenough, W. T., Black, J. E. & Wallace, C. S. Experience and brain development. Child Dev. 58, 539–559 (2022).
McKone, E., Kanwisher, N. & Duchaine, B. C. Can generic expertise explain special processing for faces? Trends Cogn. Sci. 11, 8–15 (2007).
Sur, M., Garraghty, P. E. & Roe, A. W. Experimentally induced visual projections into auditory thalamus and cortex. Science 242, 1437–1441 (1988).
von Melchner, L., Pallas, S. L. & Sur, M. Visual behaviour mediated by retinal projections directed to the auditory pathway. Nature 404, 871–876 (2000).
Finlay, B. L. Endless minds most beautiful. Dev. Sci. 10, 30–34 (2007).
Gomez-Robles, A., Hopkins, W. D. & Sherwood, C. C. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc. Biol. Sci. 280, 20130575 (2013).
Havighurst, R. J. Developmental Tasks and Education (Univ. Chicago Press, 1948).
Oakes, L.M. & Rakison, D.H. Developmental Cascades: Building The Infant Mind (Oxford Univ. Press, 2020).
Smith, L. B. & Thelen, E. Development as a dynamic system. Trends Cogn. Sci. 7, 343–348 (2003).
Cashon, C. H., Ha, O. R., Allen, C. L. & Barna, A. C. A U-shaped relation between sitting ability and upright face processing in infants. Child Dev. 84, 802–809 (2013).
Goren, C. C., Sarty, M. & Wu, P. Y. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 56, 544–549 (1975).
Johnson, M. H., Dziurawiec, S., Ellis, H. & Morton, J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19 (1991).
Valenza, E., Simion, F., Cassia, V. M. & Umiltà, C. Face preference at birth. J. Exp. Psychol. Hum. Percept. Perform. 22, 892–903 (1996).
Dobson, V. & Teller, D. Y. Visual acuity in human infants: a review and comparison of behavioral and electrophysiological studies. Vis. Res. 18, 1469–1483 (1978).
Atkinson, J., Braddick, O. & Braddick, F. Acuity and contrast sensivity of infant vision. Nature 247, 403–404 (1974).
Kiorpes, L. The puzzle of visual development: behavior and neural limits. J. Neurosci. 36, 11384–11393 (2016).
Kiorpes, L. & Movshon, J. in The New Visual Neurosciences Ch. 12 (eds Chalupa, L. & Werner, J. S.) 1423–1431 (MIT Press, 2003).
Kleiner, K. A. & Banks, M. S. Stimulus energy does not account for 2-month-olds’ face preferences. J. Exp. Psychol. Hum. Percept. Perform. 13, 594–600 (1987).
Morton, J., Johnson, M. H. & Maurer, D. On the reasons for newborns’ responses to faces. Infant Behav. Dev. 13, 99–103 (1990).
Macchi Cassia, V., Turati, C. & Simion, F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychol. Sci. 15, 379–383 (2004).
Simion, F., Macchi Cassia, V., Turati, C. & Valenza, E. The origins of face perception: specific versus non-specific mechanisms. Infant Child Dev. 10, 59–65 (2001).
Wagemans, J. Detection of visual symmetries. Spat. Vis. 9, 9–32 (1995).
Slater, A. & Sykes, M. Newborn infants’ visual responses to square wave gratings. Child Dev. 48, 545–554 (1977).
Wilkinson, N., Paikan, A., Gredebäck, G., Rea, F. & Metta, G. Staring us in the face? An embodied theory of innate face preference. Dev. Sci. 17, 809–825 (2014).
Bushnell, I. W. R. Mother’s face recognition in newborn infants: learning and memory. Infant Child Dev. 10, 67–74 (2001).
Pascalis, O., de Schonen, S., Morton, J., Deruelle, C. & Fabre-Grenet, M. Mother’s face recognition by neonates: a replication and an extension. Infant Behav. Dev. 18, 79–85 (1995).
Sai, F. Z. The role of the mother’s voice in developing mother’s face preference: evidence for intermodal perception at birth. Infant Child Dev. 14, 29–50 (2005).
Lewkowicz, D. J. Infant perception of audio-visual speech synchrony. Dev. Psychol. 46, 66–77 (2010).
Lewkowicz, D. J. & Lickliter, R. The development of intersensory perception: comparative perspectives. J. Cogn. Neurosci. 8, 185–187 (1996).
Slater, A., Brown, E. & Badenoch, M. Intermodal perception at birth: newborn infants’ memory for arbitrary auditory–visual pairings. Early Dev. Parent. 6, 99–104 (1997).
Fausey, C. M., Jayaraman, S. & Smith, L. B. From faces to hands: changing visual input in the first two years. Cognition 152, 101–107 (2016).
Xiao, N. G. et al. Eye tracking reveals a crucial role for facial motion in recognition of faces by infants. Dev. Psychol. 51, 744–757 (2015).
Eisenberg, N., Cumberland, A. & Spinrad, T. L. Parental socialization of emotion. Psychol. Inq. 9, 241–273 (1998).
Dissanayake, E. Motherese is but one part of a ritualized, multimodal, temporally organized, affiliative interaction. Behav. Brain Sci. 27, 491–503 (2004).
Sugden, N. A. & Moulson, M. C. Hey baby, what’s “up”? One- and 3-month-olds experience faces primarily upright but non-upright faces offer the best views. Q. J. Exp. Psychol. 70, 959–969 (2017).
Rennels, J. L. & Davis, R. E. Facial experience during the first year. Infant Behav. Dev. 31, 665–678 (2008).
Sugden, N. A., Mohamed-Ali, M. I. & Moulson, M. C. I spy with my little eye: typical, daily exposure to faces documented from a first-person infant perspective: infants’ daily exposure to faces. Dev. Psychobiol. 56, 249–261 (2014).
Liu, S. et al. Asian infants show preference for own-race but not other-race female faces: the role of infant caregiving arrangements. Front. Psychol. 6, 593 (2015).
Sugita, Y. Face perception in monkeys reared with no exposure to faces. Proc. Natl Acad. Sci. USA 105, 394–398 (2008).
Smith, L. B. & Slone, L. K. A developmental approach to machine learning? Front. Psychol. 8, 2124 (2017).
Abudarham, N., Grosbard, I. & Yovel, G. Face recognition depends on specialized mechanisms tuned to view-invariant facial features: insights from deep neural networks optimized for face or object recognition. Cogn. Sci. 45, e13031 (2021).
Földiák, P. Learning invariance from transformation sequences. Neural Comput. 3, 194–200 (1991).
Li, N. & DiCarlo, J. J. Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science 321, 1502–1507 (2008).
Wiskott, L. & Sejnowski, T. J. Slow feature analysis: unsupervised learning of invariances. Neural Comput. 14, 715–770 (2002).
Wood, J. N. & Wood, S. M. W. The development of newborn object recognition in fast and slow visual worlds. Proc. R. Soc. B 283, 20160166 (2016).
Flom, R. Perceptual narrowing: retrospect and prospect: perceptual narrowing. Dev. Psychobiol. 56, 1442–1453 (2014).
Lewkowicz, D. J. & Ghazanfar, A. A. The emergence of multisensory systems through perceptual narrowing. Trends Cogn. Sci. 13, 470–478 (2009).
Maurer, D. & Werker, J. F. Perceptual narrowing during infancy: a comparison of language and faces: language and faces. Dev. Psychobiol. 56, 154–178 (2014).
Pascalis, O. et al. On the links among face processing, language processing, and narrowing during development. Child Dev. Perspect. 8, 65–70 (2014).
Simpson, E. A., Jakobsen, K. V., Fragaszy, D. M., Okada, K. & Frick, J. E. The development of facial identity discrimination through learned attention. Dev. Psychobiol. 56, 1083–1101 (2014).
Kelly, D. J. et al. Development of the other-race effect during infancy: evidence toward universality? J. Exp. Child Psychol. 104, 105–114 (2009).
Kelly, D. J. et al. The other-race effect develops during infancy: evidence of perceptual narrowing. Psychol. Sci. 18, 1084–1089 (2007).
Pascalis, O. et al. Plasticity of face processing in infancy. Proc. Natl Acad. Sci. USA 102, 5297–5300 (2005).
Scott, L. S. & Monesson, A. The origin of biases in face perception. Psychol. Sci. 20, 676–680 (2009).
Vogel, M., Monesson, A. & Scott, L. S. Building biases in infancy: the influence of race on face and voice emotion matching. Dev. Sci. 15, 359–372 (2012).
Anzures, G., Quinn, P. C., Pascalis, O., Slater, A. M. & Lee, K. Development of own-race biases. Vis. Cogn. 21, 1165–1182 (2013).
Quinn, P. C., Lee, K., Pascalis, O. & Tanaka, J. W. Narrowing in categorical responding to other-race face classes by infants. Dev. Sci. 19, 362–371 (2016).
Bar-Haim, Y., Ziv, T., Lamy, D. & Hodes, R. M. Nature and nurture in own-race face processing. Psychol. Sci. 17, 159–163 (2006).
Balas, B. & Saville, A. Hometown size affects the processing of naturalistic face variability. Vis. Res. 141, 228–236 (2017).
Pickron, C. B., Fava, E. & Scott, L. S. Follow my gaze: face race and sex influence gaze-cued attention in infancy. Infancy 22, 626–644 (2017).
Xiao, N. G. et al. Older but not younger infants associate own-race faces with happy music and other-race faces with sad music. Dev. Sci. 21, e12537 (2018).
Groves, P. M. & Thompson, R. F. Habituation: a dual-process theory. Psychol. Rev. 77, 419–450 (1970).
Morand-Ferron, J. Why learn? The adaptive value of associative learning in wild populations. Curr. Opin. Behav. Sci. 16, 73–79 (2017).
Rakison, D. H. & Yermolayeva, Y. How to identify a domain-general learning mechanism when you see one. J. Cogn. Dev. 12, 134–153 (2011).
Thelen, E. & Smith, L. B. A Dynamic Systems Approach to The Development of Cognition and Action (MIT Press, 1994).
Little, A. H., Lipsitt, L. P. & Rovee-Collier, C. Classical conditioning and retention of the infant’s eyelid response: effects of age and interstimulus interval. J. Exp. Child Psychol. 37, 512–524 (1984).
Mundy, P. & Newell, L. Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 16, 269–274 (2007).
Pelaez, M. & Monlux, K. Operant conditioning methodologies to investigate infant learning. Eur. J. Behav. Anal. 18, 212–241 (2017).
Pelaez, M. & Monlux, K. Development of communication in infants: implications for stimulus relations research. Perspect. Behav. Sci. 41, 175–188 (2018).
Peláez-Nogueras, M. et al. Infants’ preference for touch stimulation in face-to-face interactions. J. Appl. Dev. Psychol. 17, 199–213 (1996).
Suarez-Rivera, C., Smith, L. B. & Yu, C. Multimodal parent behaviors within joint attention support sustained attention in infants. Dev. Psychol. 55, 96–109 (2019).
Aslin, R. N. Statistical learning: a powerful mechanism that operates by mere exposure. Wiley Interdiscip. Rev. Cogn. Sci. 8, e1373 (2017).
Fiser, J. & Aslin, R. N. Statistical learning of new visual feature combinations by infants. Proc. Natl Acad. Sci. USA 99, 15822–15826 (2002).
Dotsch, R., Hassin, R. & Todorov, A. Statistical learning shapes face evaluation. Nat. Hum. Behav. 1, 0001 (2016).
Altvater-Mackensen, N., Jessen, S. & Grossmann, T. Brain responses reveal that infants’ face discrimination is guided by statistical learning from distributional information. Dev. Sci. 20, e12393 (2017).
Jessen, S. & Grossmann, T. Exploring the role of spatial frequency information during neural emotion processing in human infants. Front. Hum. Neurosci. 11, 486 (2017).
Pickron, C. B., Iyer, A., Fava, E. & Scott, L. S. Learning to individuate: the specificity of labels differentially impacts infant visual attention. Child Dev. 89, 698–710 (2018).
Scott, L. S. Mechanisms underlying the emergence of object representations during infancy. J. Cogn. Neurosci. 23, 2935–2944 (2011).
Scott, L. S. & Monesson, A. Experience-dependent neural specialization during infancy. Neuropsychologia 48, 1857–1861 (2010).
Dewar, K. & Xu, F. Do 9-month-old infants expect distinct words to refer to kinds? Dev. Psychol. 43, 1227–1238 (2007).
Halberda, J., Mazzocco, M. M. M. & Feigenson, L. Individual differences in non-verbal number acuity correlate with maths achievement. Nature 455, 665–668 (2008).
Xu, Y. et al. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644 (2005).
Rankin, C. H. et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138 (2009).
Kaplan, P. S. & Werner, J. S. Habituation, response to novelty, and dishabituation in human infants: tests of a dual-process theory of visual attention. J. Exp. Child Psychol. 42, 199–217 (1986).
Kaplan, P., Werner, J. & Rudy, J. Habituation, sensitization, and infant visual attention. Adv. Infancy Res. 6, 61–109 (1990).
Kavšek, M. The comparator model of infant visual habituation and dishabituation: recent insights: infant visual habituation and dishabituation. Dev. Psychobiol. 55, 793–808 (2013).
Colombo, J. Infant attention grows up: the emergence of a developmental cognitive neuroscience perspective. Curr. Dir. Psychol. Sci. 11, 196–200 (2002).
Aslin, R. N. What’s in a look? Dev. Sci. 10, 48–53 (2007).
Colombo, J. & Mitchell, D. W. Infant visual habituation. Neurobiol. Learn. Mem. 92, 225–234 (2009).
Lipsitt, L. P. Learning, habituation, and classical conditioning processes in the human newborn: sensitization. Ann. NY Acad. Sci. 608, 113–127 (1990).
Kavšek, M. & Bornstein, M. H. Visual habituation and dishabituation in preterm infants: a review and meta-analysis. Res. Dev. Disabil. 31, 951–975 (2010).
Snyder, K., Webb, S. J. & Nelson, C. A. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behav. Dev. 25, 466–494 (2002).
Colombo, J., Frick, J. E. & Gorman, S. A. Sensitization during visual habituation sequences: procedural effects and individual differences. J. Exp. Child Psychol. 67, 223–235 (1997).
Bell, A. H., Hadj-Bouziane, F., Frihauf, J. B., Tootell, R. B. & Ungerleider, L. G. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J. Neurophysiol. 101, 688–700 (2009).
Clark, V. P. et al. Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography. Neuroimage 4, 1–15 (1996).
Jacques, C. et al. Corresponding ECoG and fMRI category-selective signals in human ventral temporal cortex. Neuropsychologia 83, 14–28 (2016).
Kanwisher, N., McDermott, J. & Chun, M. M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
Pinsk, M. A. et al. Neural representations of faces and body parts in macaque and human cortex: a comparative fMRI study. J. Neurophysiol. 101, 2581–2600 (2009).
Puce, A., Allison, T., Asgari, M., Gore, J. C. & McCarthy, G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J. Neurosci. 16, 5205–5215 (1996).
Tsao, D. Y., Freiwald, W. A., Knutsen, T. A., Mandeville, J. B. & Tootell, R. B. H. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 6, 989–995 (2003).
Tsao, D. Y., Freiwald, W. A., Tootell, R. B. H. & Livingstone, M. S. A cortical region consisting entirely of face-selective cells. Science 311, 670–674 (2006).
Powell, L. J., Kosakowski, H. L. & Saxe, R. Social origins of cortical face areas. Trends Cogn. Sci. 22, 752–763 (2018).
Huber, E. et al. A lack of experience-dependent plasticity after more than a decade of recovered sight. Psychol. Sci. 26, 393–401 (2015).
Blauch, N. M., Behrmann, M. & Plaut, D. A connectivity-constrained computational account of topographic organization in primate high-level visual cortex. Proc. Natl Acad. Sci. USA 119, 1–12 (2022).
Butts, D. A. Retinal waves: implications for synaptic learning rules during development. Neuroscientist 8, 243–253 (2002).
Hebb, D. O. The organization of behavior: a neuropsychological theory (John Wiley and Sons, 1949). Sci. Educ. 34, 336–337 (1950).
Arcaro, M. J., Schade, P. F. & Livingstone, M. S. Universal mechanisms and the development of the face network: what you see is what you get. Annu. Rev. Vis. Sci. 5, 341–372 (2019).
Dubois, J. et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276, 48–71 (2014).
Li, J., Osher, D. E., Hansen, H. A. & Saygin, Z. M. Innate connectivity patterns drive the development of the visual word form area. Sci. Rep. 10, 18039 (2020).
Goldman-Rakic, P. S. Development of cortical circuitry and cognitive function. Child Dev. 58, 601–622 (1987).
Garcia, K. E. et al. Dynamic patterns of cortical expansion during folding of the preterm human brain. Proc. Natl Acad. Sci. USA 115, 3156–3161 (2018).
Hilgetag, C. C. & Barbas, H. Developmental mechanics of the primate cerebral cortex. Anat. Embryol. 210, 411–417 (2005).
Arcaro, M. J. & Livingstone, M. S. A hierarchical, retinotopic proto-organization of the primate visual system at birth. eLife 6, e26196 (2017).
Arcaro, M. J., Schade, P. F. & Livingstone, M. S. Body map proto-organization in newborn macaques. Proc. Natl Acad. Sci. USA 116, 24861–24871 (2019).
Ellis, C. T. et al. Retinotopic organization of visual cortex in human infants. Neuron 109, 2616–2626.e6 (2021).
Souther, A., & Banks, M. The human face: a view from the infant’s eye. Presented at the meeting of the Society for Research in Child Development (1979).
Kleiner, K. A. & Banks, M. S. Stimulus energy does not account for 2-month-olds’ face preferences. J. Exp. Psychol. Hum. Percept. Perform. 13, 594–600 (1987).
Simion, F., Valenza, E., Cassia, V. M., Turati, C. & Umilta, C. Newborns’ preference for up-down asymmetrical configurations. Dev. Sci. 5, 427–434 (2002).
Turati, C., Simion, F., Milani, I. & Umiltà, C. Newborns’ preference for faces: what is crucial? Dev. Psychol. 38, 875–882 (2002).
Derrington, A. M. & Lennie, P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J. Physiol. 357, 219–240 (1984).
Liu, C.-S. J. et al. Magnocellular and parvocellular visual pathways have different blood oxygen level-dependent signal time courses in human primary visual cortex. Am. J. Neuroradiol. 27, 1628–1634 (2006).
Bourne, J. A. & Rosa, M. G. P. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT). Cereb. Cortex 16, 405–414 (2006).
Rakic, P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J. Comp. Neurol. 176, 23–52 (1977).
Hammarrenger, B. et al. Magnocellular and parvocellular developmental course in infants during the first year of life. Doc. Ophthalmol. 107, 225–233 (2003).
Atkinson, J. Early visual development: differential functioning of parvocellular and magnocellular pathways. Eye 6, 129–135 (1992).
Rodman, H. R., Skelly, J. P. & Gross, C. G. Stimulus selectivity and state dependence of activity in inferior temporal cortex of infant monkeys. Proc. Natl Acad. Sci. USA 88, 7572–7575 (1991).
Rodman, H. R., Scalaidhe, S. P. & Gross, C. G. Response properties of neurons in temporal cortical visual areas of infant monkeys. J. Neurophysiol. 70, 1115–1136 (1993).
Distler, C., Bachevalier, J., Kennedy, C., Mishkin, M. & Ungerleider, L. G. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb. Cortex 6, 184–195 (1996).
Pitti, A., Kuniyoshi, Y., Quoy, M. & Gaussier, P. Modeling the minimal newborn’s intersubjective mind: the visuotopic-somatotopic alignment hypothesis in the superior colliculus. PLoS ONE 8, e69474 (2013).
Robinson, D. L. & Petersen, S. E. The pulvinar and visual salience. Trends Neurosci. 15, 127–132 (1992).
Stepniewska, I., Qi, H.-X. & Kaas, J. H. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 17, 529–549 (2000).
Homman-Ludiye, J., Kwan, W. C., de Souza, M. J. & Bourne, J. A. Full: Ontogenesis and development of the nonhuman primate pulvinar. J. Comp. Neurol. 526, 2870–2883 (2018).
Nguyen, M. N. et al. Population coding of facial information in the monkey superior colliculus and pulvinar. Front. Neurosci. 10, 583 (2016).
Arcaro, M. J. & Livingstone, M. S. On the relationship between maps and domains in inferotemporal cortex. Nat. Rev. Neurosci. 22, 573–583 (2021).
Kwon, M.-K., Setoodehnia, M., Baek, J., Luck, S. J. & Oakes, L. M. The development of visual search in infancy: attention to faces versus salience. Dev. Psychol. 52, 537–555 (2016).
Hasson, U., Levy, I., Behrmann, M., Hendler, T. & Malach, R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron 34, 479–490 (2002).
Lafer-Sousa, R. & Conway, B. R. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat. Neurosci. 16, 1870–1878 (2013).
Kamps, F. S., Hendrix, C. L., Brennan, P. A. & Dilks, D. D. Connectivity at the origins of domain specificity in the cortical face and place networks. Proc. Natl Acad. Sci. USA 117, 6163–6169 (2020).
Xu, R., Bichot, N. P., Takahashi, A. & Desimone, R. The cortical connectome of primate lateral prefrontal cortex. Neuron 110, 312–327.e7 (2022).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Groen, I. I. A., Dekker, T. M., Knapen, T. & Silson, E. H. Visuospatial coding as ubiquitous scaffolding for human cognition. Trends Cogn. Sci. 26, 81–96 (2022).
Triplett, J. W., Phan, A., Yamada, J. & Feldheim, D. A. Alignment of multimodal sensory input in the superior colliculus through a gradient-matching mechanism. J. Neurosci. 32, 5264–5271 (2012).
Tomasello, R., Wennekers, T., Garagnani, M. & Pulvermüller, F. Visual cortex recruitment during language processing in blind individuals is explained by Hebbian learning. Sci. Rep. 9, 3579 (2019).
Murty, R. N. A. et al. Visual experience is not necessary for the development of face-selectivity in the lateral fusiform gyrus. Proc. Natl Acad. Sci. USA 117, 23011–23020 (2020).
van den Hurk, J., Van Baelen, M. & Op de Beeck, H. P. Development of visual category selectivity in ventral visual cortex does not require visual experience. Proc. Natl Acad. Sci. USA 114, E4501–E4510 (2017).
Tzourio-Mazoyer, N. et al. Neural correlates of woman face processing by 2-month-old infants. Neuroimage 15, 454–461 (2002).
Srihasam, K., Vincent, J. L. & Livingstone, M. S. Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat. Neurosci. 17, 1776–1783 (2014).
Yue, X., Robert, S. & Ungerleider, L. G. Curvature processing in human visual cortical areas. Neuroimage 222, 117295 (2020).
Long, B., Yu, C.-P. & Konkle, T. Mid-level visual features underlie the high-level categorical organization of the ventral stream. Proc. Natl Acad. Sci. USA 115, E9015–E9024 (2018).
Bentin, S., Allison, T., Puce, A., Perez, E. & McCarthy, G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 8, 551–565 (1996).
Caldara, R. et al. Face versus non-face object perception and the ‘other-race’ effect: a spatio-temporal event-related potential study. Clin. Neurophysiol. 114, 515–528 (2003).
Itier, R. J., Latinus, M. & Taylor, M. J. Face, eye and object early processing: what is the face specificity? Neuroimage 29, 667–676 (2006).
Conte, S., Richards, J. E., Guy, M. W., Xie, W. & Roberts, J. E. Face-sensitive brain responses in the first year of life. Neuroimage 211, 116602 (2020).
Guy, M. W., Zieber, N. & Richards, J. E. The cortical development of specialized face processing in infancy. Child Dev. 87, 1581–1600 (2016).
de Haan, M., Pascalis, O. & Johnson, M. H. Specialization of neural mechanisms underlying face recognition in human infants. J. Cogn. Neurosci. 14, 199–209 (2002).
Halit, H., de Haan, M. & Johnson, M. H. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage 19, 1180–1193 (2003).
Balas, B., Westerlund, A., Hung, K. & Nelson Iii, C. A. Shape, color and the other-race effect in the infant brain. Dev. Sci. 14, 892–900 (2011).
Scott, L. S. & Nelson, C. A. Featural and configural face processing in adults and infants: a behavioral and electrophysiological investigation. Perception 35, 1107–1128 (2006).
Righi, G., Westerlund, A., Congdon, E. L., Troller-Renfree, S. & Nelson, C. A. Infants’ experience-dependent processing of male and female faces: insights from eye tracking and event-related potentials. Dev. Cogn. Neurosci. 8, 144–152 (2014).
Aylward, E. H. et al. Brain activation during face perception: evidence of a developmental change. J. Cogn. Neurosci. 17, 308–319 (2005).
Passarotti, A. M. et al. The development of face and location processing: an fMRI study. Dev. Sci. 6, 100–117 (2003).
Cantlon, J. F., Pinel, P., Dehaene, S. & Pelphrey, K. A. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex 21, 191–199 (2011).
Leibo, J. Z., Liao, Q., Anselmi, F. & Poggio, T. The invariance hypothesis implies domain-specific regions in visual cortex. PLoS Comput. Biol. 11, e1004390 (2015).
Golarai, G. et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 10, 512–522 (2007).
Bukach, C. M., Gauthier, I. & Tarr, M. J. Beyond faces and modularity: the power of an expertise framework. Trends Cogn. Sci. 10, 159–166 (2006).
Carey, S. & Diamond, R. Are faces perceived as configurations more by adults than by children? Vis. Cogn. 1, 253–274 (1994).
Bate, S. & Bennetts, R. J. The rehabilitation of face recognition impairments: a critical review and future directions. Front. Hum. Neurosci. 8, 491 (2014).
Scott, L. S. & Brito, N. H. Supporting healthy brain and behavioral development during infancy. Policy Insights Behav. Brain Sci. 9, 129–136 (2022).
Grill-Spector, K., Weiner, K. S., Kay, K. & Gomez, J. The functional neuroanatomy of human face perception. Annu. Rev. Vis. Sci. 3, 167–196 (2017).
Gruart, A., Leal-Campanario, R., López-Ramos, J. C. & Delgado-García, J. M. Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 124, 3–18 (2015).
Zeithamova, D. et al. Brain mechanisms of concept learning. J. Neurosci. 39, 8259–8266 (2019).
Turk-Browne, N. B., Scholl, B. J. & Chun, M. M. Babies and brains: habituation in infant cognition and functional neuroimaging. Front. Hum. Neurosci. 2, 16 (2008).
Grill-Spector, K., Henson, R. & Martin, A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23 (2006).
Krekelberg, B., Boynton, G. M. & van Wezel, R. J. A. Adaptation: from single cells to BOLD signals. Trends Neurosci 29, 250–256 (2006).
Wheatley, T., Weisberg, J., Beauchamp, M. S. & Martin, A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J. Cogn. Neurosci. 17, 1871–1885 (2005).
Webster, M. A. & MacLeod, D. I. A. Visual adaptation and face perception. Phil. Trans. R. Soc. B 366, 1702–1725 (2011).
Jiang, F., Blanz, V. & O’Toole, A. J. Three-dimensional information in face representations revealed by identity aftereffects. Psychol. Sci. 20, 318–325 (2009).
Leopold, D. A., O’Toole, A. J., Vetter, T. & Blanz, V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat. Neurosci. 4, 89–94 (2001).
Barry-Anwar, R., Riggins, T. & Scott, L. S. in The Oxford Handbook of Developmental Cognitive Neuroscience (ed. Cohen Kadosh, K.) (Oxford Univ. Press, 2020).
Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617 (2012).
Hoehl, S., Michel, C., Reid, V. M., Parise, E. & Striano, T. Eye contact during live social interaction modulates infants’ oscillatory brain activity. Soc. Neurosci. 9, 300–308 (2014).
Michel, C. et al. Theta- and alpha-band EEG activity in response to eye gaze cues in early infancy. Neuroimage 118, 576–583 (2015).
Snyder, K. A. & Keil, A. Repetition suppression of induced gamma activity predicts enhanced orienting toward a novel stimulus in 6-month-old infants. J. Cogn. Neurosci. 20, 2137–2152 (2008).
McCarthy, G., Puce, A., Gore, J. C. & Allison, T. Face-specific processing in the human fusiforrn gyms. J. Cogn. Neurosci. 9, 605–610 (1997).
Allison, T. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb. Cortex 9, 415–430 (1999).
Rossion, B. et al. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126, 2381–2395 (2003).
Behrmann, M. & Plaut, D. C. A vision of graded hemispheric specialization. Ann. NY Acad. Sci. 1359, 30–46 (2015).
Dehaene, S. & Cohen, L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 15, 254–262 (2011).
Dundas, E. M., Plaut, D. C. & Behrmann, M. The joint development of hemispheric lateralization for words and faces. J. Exp. Psychol. Gen. 142, 348–358 (2013).
Hildesheim, F. E. et al. The trajectory of hemispheric lateralization in the core system of face processing: a cross-sectional functional magnetic resonance imaging pilot study. Front. Psychol. 11, 507199 (2020).
Scott, L. S., Shannon, R. W. & Nelson, C. A. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy 10, 171–186 (2006).
de Haan, M. & Nelson, C. A. Brain activity differentiates face and object processing in 6-month-old infants. Dev. Psychol. 35, 1113–1121 (1999).
Gliga, T. & Dehaene-Lambertz, G. Development of a view-invariant representation of the human head. Cognition 102, 261–288 (2007).
Norcia, A. M., Appelbaum, L. G., Ales, J. M., Cottereau, B. R. & Rossion, B. The steady-state visual evoked potential in vision research: a review. J. Vis. 15, 4 (2015).
Barry-Anwar, R., Hadley, H., Conte, S., Keil, A. & Scott, L. S. The developmental time course and topographic distribution of individual-level monkey face discrimination in the infant brain. Neuropsychologia 108, 25–31 (2018).
de Heering, A. & Rossion, B. Rapid categorization of natural face images in the infant right hemisphere. eLife 4, e06564 (2015).
Farzin, F., Hou, C. & Norcia, A. M. Piecing it together: infants’ neural responses to face and object structure. J. Vis. 12, 6–6 (2012).
Peykarjou, S., Hoehl, S., Pauen, S. & Rossion, B. Rapid categorization of human and ape faces in 9-month-old infants revealed by fast periodic visual stimulation. Sci. Rep. 7, 12526 (2017).
Acknowledgements
The authors thank members of the University of Florida’s Brain, Cognition and Developmental Laboratory for relevant discussions. Funding for this work was provided by the National Science Foundation to L.S.S. (BCS-1728133 and BCS-1056805/BCS-1560810).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Psychology thanks Margaret Moulson, Marlene Behrmann and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scott, L.S., Arcaro, M.J. A domain-relevant framework for the development of face processing. Nat Rev Psychol 2, 183–195 (2023). https://doi.org/10.1038/s44159-023-00152-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44159-023-00152-5