Abstract
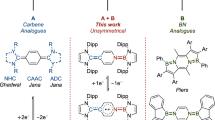
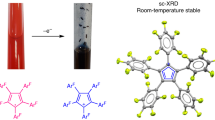
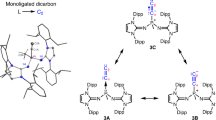
Stable tertiary R3C· carbon radicals have been known since Gomberg’s pioneering discovery of the triphenylmethyl radical more than a century ago. In stark contrast, secondary R2CH· and primary RCH2· carbon radicals are elusive species only observed spectroscopically. Herein, we describe the isolation of a crystalline pentadienyl-type radical, featuring a central secondary carbon, prepared by single-electron reduction of a bis(imino)carbene conjugate acid. The key to its stability is the presence of two N-heterocyclic imine substituents, which impart both steric protection and electronic stabilization. Density functional theory calculations confirm that the central secondary carbon atom is the principal spin carrier. Accordingly, electron paramagnetic resonance spectroscopy reveals that the hydrogen atom attached to the central carbon atom exhibits an exceptionally large hyperfine coupling constant (>10 G), which we believe is the largest recorded for an isolated organic radical. In the presence of an H· donor, hydrogen atom abstraction occurs exclusively at the central carbon to form a methylene unit. Furthermore, this radical can participate in a radical–radical cross-coupling reaction with [(2,2,6,6-tetramethylpiperidin-1-yl)oxyl], providing an example of cross-coupling between two stable organic radicals.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2245614 (1H+[TfO−]), 2267005 (1H∙), 2267006 (1H2) and 2312276 (1H-TEMPO). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Gomberg, M. An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 22, 757–771 (1900).
Chen, Z. X., Li, Y. & Huang, F. Persistent and stable organic radicals: design, synthesis, and applications. Chem 7, 288–332 (2021).
Fessenden, R. W. & Schuler, R. H. Electron spin resonance studies of transient alkyl radicals. J. Chem. Phys. 39, 2147–2195 (1963).
Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds (CRC, 2002).
Stubbe, J. & Nocera, D. G. Radicals in biology: your life is in their hands. J. Am. Chem. Soc. 143, 13463–13472 (2021).
Broderick, J. B., Duffus, B. R., Duschene, K. S. & Shepard, E. M. Radical S‑adenosylmethionine enzymes. Chem. Rev. 114, 4229–4317 (2014).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Nesterov, V. et al. NHCs in main group chemistry. Chem. Rev. 118, 9678–9842 (2018).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Kim, H. & Lee, E. Ambiphilic singlet carbenes: electron donors and acceptors. Bull. Korean Chem. Soc. 43, 1328–1341 (2022).
Kushvaha, S. K., Mishra, A., Roesky, H. W. & Mondal, K. C. Recent advances in the domain of cyclic (alkyl)(amino) carbenes. Chem. Asian J. 17, e202101301 (2022).
Melaimi, M., Jazzar, R., Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): recent developments. Angew. Chem. Int. Ed. 56, 10046–10068 (2017).
Kim, Y. & Lee, E. Stable organic radicals derived from N-heterocyclic carbenes. Chem. Eur. J. 24, 19110–19121 (2018).
Kundu, S., Sinhababu, S., Chandrasekhar, V. & Roesky, H. W. Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents. Chem. Sci. 10, 4727–4741 (2019).
Martin, C. D., Soleilhavoup, M. & Bertrand, G. Carbene-stabilized main group radicals and radical ions. Chem. Sci. 4, 3020–3030 (2013).
Gorodetsky, B., Ramnial, T., Branda N. R. & Clyburne, J. A. C. Electrochemical reduction of an imidazolium cation: a convenient preparation of imidazol-2-ylidenes and their observation in an ionic liquid. Chem. Commun. https://doi.org/10.1039/B407386J (2004).
Das, A., Ahmed, J., Rajendran, N. M., Adhikari, D. & Mandal, S. K. A bottleable imidazole-based radical as a single electron transfer reagent. J. Org. Chem. 86, 1246–1252 (2021).
Aldeco-Perez, E. et al. Isolation of a C-5-deprotonated imidazolium, a crystalline “abnormal” N-heterocyclic carbene. Science 326, 556–559 (2009).
Sau, S. C., Hota, P. K., Mandal, S. K., Soleilhavoup, M. & Bertrand, G. Stable abnormal N-heterocyclic carbenes and their applications. Chem. Soc. Rev. 249, 1233–1252 (2020).
Loh, Y. K., Melaimi, M., Munz, D. & Bertrand, G. An air-stable “masked” bis(imino)carbene: a carbon-based dual ambiphile. J. Am. Chem. Soc. 145, 2064–2069 (2023).
Loh, Y. K., Melaimi, M., Gembicky, M., Munz, D. & Bertrand, G. A crystalline doubly oxidized carbene. Nature 623, 66–70 (2023).
Ochiai, T., Franz, D. & Inoue, S. Applications of N-heterocyclic imines in main group chemistry. Chem. Soc. Rev. 45, 6327–6344 (2016).
Goettel, J. T., Gao, H., Dotzauer, S. & Braunschweig, H. MeCAAC=N–: a cyclic (alkyl)(amino)carbene imino ligand. Chem. Eur. J. 26, 1136–1143 (2020).
Huynh, S. et al. Cyclic alkyl(amino)iminates (CAAIs) as strong 2σ,4π-electron donor ligands for the stabilisation of boranes and diboranes(4): a synthetic and computational study. Dalton Trans. 52, 3869–3876 (2023).
Mahoney, J. K. et al. Air-persistent monomeric (amino)(carboxy) radicals derived from cyclic (alkyl)(amino) carbenes. J. Am. Chem. Soc. 137, 7519–7525 (2015).
Styra, S. et al. Crystalline cyclic (alkyl)(amino)carbene-tetrafluoropyridyl radical. Chem. Eur. J. 21, 8441–8446 (2015).
Mahoney, J. K., Jazzar, R., Royal, G., Martin, D. & Bertrand, G. The advantages of cyclic over acyclic carbenes to access isolable capto-dative C-centered radicals. Chem. Eur. J. 23, 6206–6212 (2017).
Hansmann, M. M., Melaimi, M. & Bertrand, G. Crystalline monomeric allenyl/propargyl radical. J. Am. Chem. Soc. 139, 15620–15623 (2017).
Regnier, V. et al. What are the radical intermediates in oxidative N‑heterocyclic carbene organocatalysis? J. Am. Chem. Soc. 141, 1109–1117 (2019).
Murphy, J. A. Discovery and development of organic super-electron-donors. J. Org. Chem. 79, 3731–3746 (2014).
Doni, E. & Murphy, J. A. Evolution of neutral organic super-electrondonors and their applications. Chem. Commun. 50, 6073–6087 (2014).
Dalton, D. R. & Liebman, S. A. Electron spin resonance studies on neutral aromatic hydrocarbon radicals. J. Am. Chem. Soc. 91, 1194–1199 (1969).
Mohos, B., Tüdös, F. & Jókay, L. ESR investigation of the triphenylmethyl radical. Phys. Lett. A 24, 311–312 (1967).
Welle, F. M., Beckhaus, H.-D. & Rüchardt, C. Thermochemical stability of alpha-amino-alpha-carbonylmethyl radicals and their resonance as measured by ESR. J. Org. Chem. 62, 552–558 (1997).
Griller, D., Ingold, K. U. & Walton, J. C. Two conformations of the pentadienyl radical. J. Am. Chem. Soc. 101, 758–759 (1979).
Breitwieser, K., Bahmann, H., Weiss, R. & Munz, D. Gauging radical stabilization with carbenes. Angew. Chem. Int. Ed. 61, e202206390 (2022).
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Jeffrey, J. L., Petronijević, F. R. & MacMillan, D. W. C. Selective radical–radical cross-couplings: design of a formal β‑Mannich reaction. J. Am. Chem. Soc. 137, 8404–8407 (2015).
Lin, Q., Dawson, G. & Diao, T. Experimental electrochemical potentials of nickel complexes. Synlett 32, 1606–1620 (2021).
Azevedo, F., Freire, C. & de Castro, B. Reductive electrochemical study of Ni(II) complexes with N2O2 Schiff base complexes and spectroscopic characterization of the reduced species. Reactivity towards CO. Polyhedron 21, 1695–1705 (2002).
Acknowledgements
This work was supported by the NSF (grant no. CHE-2246948). Y.K.L. thanks A*STAR for the award of a postdoctoral fellowship. We gratefully acknowledge the scientific support and high-performance computing resources provided by the Erlangen National High Performance Computing Center (NHR@FAU) of the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). The hardware is funded by the German Research Foundation (DFG). EPR work was funded by NSF MRI grant no. CHE 2019066.
Author information
Authors and Affiliations
Contributions
Y.K.L. conceived and performed the synthetic experiments. L.G. carried out the UV–Vis analysis and additional synthetic experiments during revision. M.M. performed the EPR and electrochemical studies. M.M. and M.G. performed the X-ray crystallographic analyses. D.M. performed the computational work. G.B. supervised the project. The manuscript was written by Y.K.L., M.M. and G.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Figs. 1–27 and Tables 1–10.
Supplementary Data 1
Crystallographic data for compound 1H+[TfO−]; CCDC 2245614.
Supplementary Data 2
Crystallographic data for compound 1H·; CCDC 2267005.
Supplementary Data 3
Crystallographic data for compound 1H2; CCDC 2267006.
Supplementary Data 4
Crystallographic data for compound 1H-TEMPO; CCDC 2312276.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loh, Y.K., Gojiashvili, L., Melaimi, M. et al. Isolation of a pentadienyl-type radical featuring a central secondary carbon. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00516-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00516-6