Abstract
Renewable electricity-driven electrochemical nitrogen oxidation is a promising alternative to traditional Haber–Bosch and Ostwald processes to directly synthesize nitrate from nitrogen. However, its efficiency is hindered by strong competition from the oxygen evolution reaction in aqueous environments, along with a deficiency in standardized testing protocols. Here we present an efficient approach for nitrogen oxidation, substituting the oxygen evolution reaction with hydroxyl radicals (·OH) generated through hydrogen peroxide decomposition to serve as an active oxygen source. Electrochemical tests demonstrate that the nitrogen oxidation, facilitated by ·OH, can achieve a Faradaic efficiency of 25.6% and a nitrate yield of 8.3 nmol s−1 cm−2. Furthermore, we employed in situ electrochemical mass spectrometry, gas-phase infrared and electron paramagnetic resonance spectroscopy to establish a comprehensive set of benchmarks to confirm the authenticity of nitrogen activation and to examine the reaction mechanism mediated by ·OH. Techno-economic analysis underscores the promising feasibility and sustainable economic value of the presented method.

Similar content being viewed by others
Main
Nitrate is a versatile compound with broad applications in fertilizers, explosives, pharmaceuticals, dyes and emulsifiers1,2. Currently, nitrate production primarily relies on conventional, energy-intensive processes, including ammonia synthesis (Haber–Bosch method) and ammonia oxidation (Ostwald process)2,3,4. However, both methods require high-temperature and high-pressure conditions, resulting in substantial energy consumption and greenhouse gas emission. The electrochemical nitrogen oxidation reaction (NOR), driven by renewable electricity sources, has recently emerged as a promising alternative approach for nitrate synthesis5,6,7. However, the reaction efficiency is plagued by the concurrent occurrence of competing oxygen evolution reaction (OER) in aqueous solutions, which simultaneously provides active oxygen intermediates for NOR8,9.
In this Article, we circumvent the competing OER by harnessing hydroxyl radicals (·OH), which are produced through the decomposition of hydrogen peroxide (H2O2), as a potent source of active oxygen for NOR. Density functional theory (DFT) calculations revealed that ·OH (2.73 V versus normal hydrogen electrode NHE)10 holds the promising ability to surmount the thermodynamic barriers (2.23 V) associated with NOR (Fig. 1). Electrochemical tests employing Pd nanoparticles as a model catalyst demonstrated a Faradaic efficiency (FE) of 25.6% and a N2 oxidation yield to nitrate (\({{{\rm{NO}}}_{3}}^{-}\)) reaching 8.3 nmol s−1 cm−2 achieved in the presence of H2O2. Furthermore, to establish a benchmark within the realm of aqueous N2 activation, we systematically utilize closed in situ characterization techniques, continuously pushing the boundaries of detection limits to precisely assess nitrogen activation. Through the application of in situ differential electrochemical mass spectrometry (DEMS), high-resolution gas-phase Fourier transform infrared spectroscopy (HG-FTIR) and electron paramagnetic resonance (EPR) spectroscopy, we lowered the detection limit to the femtomole range and delved deeply into the mechanisms of N2 oxidation, particularly involving ·OH. Technical and economic analysis underscores that, operating on the foundation of renewable energy, the generation of H2O2 via cathodic O2 reduction, coupled with its utilization in the anodic NOR to yield \({{{\rm{NO}}}_{3}}^{-}\), leads to a viable, economically advantageous approach for \({{{\rm{NO}}}_{3}}^{-}\) production from air and renewable electricity.
a, In comparison with the oxygen source typically involved in NOR, the ·OH generated through H2O2 decomposition boasts a substantial higher oxidation potential than oxygen species derived from the water oxidation process, rendering it advantageous for NOR while circumventing OER, thus serving as a superior oxygen source. Active ·OH may also undergo surface adsorption, leading to the formation of highly active hydroxyl species compared to those generated via the OER pathway. b,c, The minimum energy path with corresponding atomic structures for H2O2 dissociation to *OH (b) and *OH diffusion (c) over Pd(111) surface. IS, initial state; TS, transition state; MS, metastable state; FS, final state. d, The free energy diagrams for the NOR towards *NO*NO over the penta-coordinated Pd sites of PdO2(110) surface with the corresponding reaction free energy and intermediate structures along the reaction pathway. The formation of *OH from both H2O and H2O2 and the complete OER pathway are considered. The grey, red, blue and white spheres represent Pd, O, N and H atoms, respectively. The energy barriers (in red) for the key kinetic reaction step are given, for which the minimum energy path with intermediate configurations are shown in Supplementary Figs. 16 and 17. The unit for energy is eV.
Results
Oxygen sources and energy barriers for NOR
The largest thermodynamic hurdle for NOR is encountered at the initial hydroxylation–deprotonation step of N2 molecules, leading to the formation of N2OH/N2O (refs. 8,9). This necessitates the prior electrical activation of H2O to yield reactive oxygen intermediates *O and *OH, followed by the application of potential to precisely guide these oxygen intermediates into NOR pathway, rather than OER7. Nonetheless, it remains exceptionally challenging to effectively steer oxygen intermediates towards the NOR pathway due to the substantial higher energy barrier needed for NOR compared with the more thermodynamically favourable OER. Merely increasing the potential to promote NOR often yields counterproductive results, as the thermodynamics favour OER11,12. Therefore, the crux of enhancing NOR efficiency lies in the discovery of a precise driving force that can selectively engage in NOR while circumventing the competitive OER, thereby serving as an oxygen source.
In nature, chemical reactions are driven by diverse forces such as light, electricity and free radicals, which are widely implicated in various efficient enzyme-catalysed reactions. Motivated by this insight, we shifted our perspective and sought to employ ·OH, known for its potent oxidative properties (2.73 V versus NHE)10, as the driving power for electrochemical NOR, capitalizing on the abundant hydrogen and oxygen constituents to serve as an optimal oxygen source while circumventing the challenges posed by the competing OER (Fig. 1a). To evaluate our hypothesis, we initially employed DFT to examine the energy barrier associated with NOR, and if lower than the oxidation potential of ·OH, it would indicate the feasibility of ·OH-driven NOR.
To bridge the theoretical and experimental aspects, we employed classically Pd single-crystal nanoparticles as model catalysts and leveraged H2O2 decomposition, generating ·OH, to examine the atomic-level reaction mechanism underlying NOR (Supplementary Fig. 1–7). It is widely acknowledged that Pd gradually undergoes partial oxidation during electrochemical oxidation, ultimately forming Pd4+, that is, PdO2 (ref. 13), a process confirmed experimentally through in situ electrochemical Raman spectroscopy (Supplementary Figs. 8 and 9). It is noteworthy that in this gradual oxidation process, besides the individual existence of Pd and PdO2, there is also a potential for their coexistence (see structural information in Supplementary Fig. 10). In the initial stage of the NOR process, we examined H2O2 and N2 adsorption on the Pd(111) surface, revealing a distinct favourability for H2O2 adsorption over N2 on the Pd(111) surface, attributed primarily to the more negative adsorption free energy of −0.14 eV for *H2O2 compared with 0.10 eV for *N2 (Supplementary Figs. 11 and 12). Due to its unlimited miscibility with H2O and the limited solubility of N2 in H2O, H2O2 readily accumulates on the Pd(111) surface before N2 adsorption, prompting our investigation into its dissociation into *OH with the minimum energy pathways shown in Fig. 1b,c. In our investigation, H2O2 is decomposed into two *OH species, with an exceptionally low energy barrier of 0.03 eV and a notably high exothermic release surpassing 1 eV (refs. 14,15). Furthermore, we noted the robust binding of *OH on the Pd(111) surface, evidenced by an adsorption free energy of approximately −1 eV (Supplementary Fig. 13). Despite the considerable binding strength of *OH, the diffusion barriers on the Pd(111) surface were notably low at just 0.16 eV (Fig. 1c), ensuring rapid diffusion and widespread coverage across both metallic and oxidized regions of Pd(111) surface. With the ease of *OH formation through H2O2 decomposition, and the strong binding and efficient diffusion over the Pd(111) surface, it is plausible to infer that *OH will preoccupy the Pd(111) surface before NOR, potentially facilitating the oxidation of N2 molecules into the ultimate product, \({{{\rm{NO}}}_{3}}^{-}\).
Subsequently, we studied the electrocatalytic activity of NOR by examining the reaction pathway, utilizing the computational hydrogen electrode (CHE) model16. It is worth noting that the electrochemical NOR leading to the final product \({{{\rm{NO}}}_{3}}^{-}\) involves crucial intermediates, particularly *NO (ref. 6), which readily transforms into \({{{\rm{NO}}}_{3}}^{-}\) through a spontaneous reaction with H2O and O2 at room temperature9,17. As such, our focus is solely on the electrochemical reaction pathway leading to the formation of *NO from N2 oxidation, initiated by the pre-adsorbed *OH. Previous studies have established *O + *N2 → *N2O and *N2O + *O → *NO*NO as pivotal kinetic steps in NOR18,19. Considering these, Supplementary Fig. 14 illustrates the energetically favourable pathway for NOR on the Pd(111) surface. The outcomes suggest that achieving efficient NOR on the Pd(111) surface poses challenges. This is primarily due to the substantial energy barrier required for *N2 oxidation by *O, inferred from the substantial endothermicity of the reaction (1.76 eV).
Therefore, building on the prior discussion, we further explore the NOR process on PdO2(110) surfaces, where *OH species readily diffuse from metallic Pd to the oxidized PdO2 area to participate in NOR. We considered two types of active site on the PdO2(110) surfaces, namely tetra-coordinated Pd and penta-coordinated Pd (Supplementary Fig. 10a). On tetra-coordinated Pd sites, the free energy diagram indicates that NOR will also be impeded by the sluggish kinetics of *N2 oxidation under ambient conditions (Supplementary Fig. 15), resembling the situation observed on Pd(111) surface. However, at the penta-coordinated Pd sites, a crucial finding emerges: the pivotal chemical step involving the formation of *N2O from co-adsorbed *O and *N2 necessitates overcoming an energy barrier of only 0.48 eV (Fig. 1d and Supplementary Fig. 16). Furthermore, it is apparent that, within the reaction pathway of the subsequent electrochemical reaction of *OH*N2O → *O*N2O, a maximum potential of ~2.2 V is required to drive this electrochemical transformation. Notably, this value is comparable to or lower than that of previous catalysts such as RuxTiyO2 (2.26 V)9, FeN4 (2.06 V) and FeN3 (3.13 V)20. To further couple *O and *N2O and generate the pivotal intermediate, *NO*NO species, there is a negative ∆G of −0.78 eV, featuring marginal energy barriers of 0.20, 0.18 and 0.12 eV throughout the transformation process (Fig. 1d and Supplementary Fig. 17). In addition, we also showed that the penta-coordinated Pd of the PdO2(110) surface has high preference for H2O2 adsorption compared with N2 (Supplementary Fig. 18), and low energy barriers for *H2O2 dissociation (0.53 eV) and *OH diffusion (0.82 eV), which further facilitate providing an oxygen source for NOR (Supplementary Fig. 19). In summary, the proposed NOR process operates via a tandem mechanism: *OH generated in the metallic Pd region migrates to the PdO2 region, where it transforms inert N2 into reactive *NO for subsequent oxidation to \({{{\rm{NO}}}_{3}}^{-}\). Notably, the onset potential of 2.23 V is lower than the oxidation potential of ·OH itself (2.73 V versus NHE). This compelling evidence provides us with both the rationale and the confidence to explore the use of ·OH as mediators for the N2-to-\({{{\rm{NO}}}_{3}}^{-}\) oxidation process.
A benchmark for excluding false positives from nitrogen
We acknowledge the substantial impact of unsubstantiated false positives on the reputation of the field of nitrogen activation in aqueous phases21,22. In our study, we aim to establish an improved and simplified standard procedure for evaluating NOR activity, employing high-resolution in situ electrochemical characterization within a controlled environment. This comprehensive approach encompasses qualitative and quantitative analyses of nitrogen-containing compounds in N2 gas, along with real-time monitoring of changes in the composition and quantities of these nitrogen-containing species under applied voltage conditions, ultimately optimizing the verification of N2 activation. Our approach encompasses testing technologies designed to incrementally enhance resolution and lower detection limits. Specifically, we employ in situ DEMS (achieving <1 ppm sensitivity), in situ electrochemical HG-FTIR (<200 ppb sensitivity) and in situ electrochemical EPR (with an absolute minimum spin number of 1.0 × 109 spin number). Together, these methodologies constitute a standardized set of procedures aimed at eliminating nitrogen contamination (see Supplementary Figs. 20–28 for details of the three in situ electrochemical testing). In comparison with established contaminant removal methods, such as copper magnet coils or commercial gas purification devices, as well as the costly and intricate 15N-labelled nitrogen source tracking techniques7, our approach is streamlined, obviating the need for gas purification devices and isotope tracking tests. Our initial focus was on both qualitatively and quantitatively assessing nitrogen impurities while confirming the activation of N2. The potentials are standardized with reversible hydrogen electrodes (RHEs). To achieve this, we devised a three-step strategy: (1) We directly scrutinize the composition and concentration of nitrogen species within commercial-grade nitrogen (99.9999% purity). (2) Employing NO as a key marker for the NOR, we apply a low voltage 1.2 V (N2(g) + 6H2O(l) → 2\({{{\rm{NO}}}_{3}}^{-}\)(aq) + 12H+(aq) + 10e−, 1.24 VSHE, −5.68 eV) to facilitate the transformation of these species into N2 (from NH3, N2H4 and NH2OH) or NO2−/\({{{\rm{NO}}}_{3}}^{-}\) (from N2O)23,24. Utilizing NO as a marker offers the distinct advantage of focusing solely on the influence of N2O, negating the necessity to account for other high-valence nitrogen pollutants such as NO2, NO2− and \({{{\rm{NO}}}_{3}}^{-}\). Moreover, given the highly exothermic nature of the N2O-to-NO conversion process, NO can be confidently employed as the exclusive marker for NOR once N2O is eliminated. (3) We apply a potential of 2.8 V, slightly higher than the oxidation potential of ·OH, to attempt to initiate N2 oxidation activation. This choice was made on the basis of the premise that successful activation of N2 at this potential could imply the feasibility of utilizing ·OH to facilitate the NOR. It is essential to note that all the aforementioned testing procedures were conducted within a closed system, ensuring that no new contaminants were introduced during the in situ testing process.
Initially, we employed in situ DEMS to assess the type and quantity of nitrogen species. Our observations revealed that the original gas contained NH3 and a small quantity of N2O, which periodically manifested as the gas circuit was sealed (Fig. 2a,b). Upon applying a potential of 1.2 V, NH3 and N2O exhibited a gradual decline until they vanished. During this process, a faintly amplified signal of NO emerged, attributed to the oxidation of N2O. Subsequently, after all signals of nitrogen species disappeared, we observed synchronous signals of N2O, NO and NO2 coinciding with the applied potential, under conditions of intermittent 2.8 V. However, it is noteworthy that their signal intensity exhibited a declining trend, attributed to the progressive oxidation of these three species. Although we successfully demonstrated NOR through in situ DEMS, it is crucial to reinforce our evidence due to the possibility of undiscovered species existing below the detection limits. To achieve this, we employed HG-FTIR spectroscopy (refer to Supplementary Figs. 24 and 25 and Supplementary Table 1 for standard gas spectra). Within the infrared spectrum, we detected a trace amount of NO, which was not clearly discerned by DEMS but manifested in the HG-FTIR spectrum. Under a constant voltage of 1.2 V, the signals of N2O, NO and NH3 gradually dissipated, leaving only a singular H2O spectrum (Fig. 2c). Conversely, under a constant voltage of 2.8 V, N2O and NO gradually emerged, and after 1.5 h, their intensities stabilized, indicating the establishment of a steady state in which N2 oxidation produces NO. Notably, the H2O peak remained consistently present throughout the test, maintaining a stable intensity. Additionally, we employed electrochemical in situ attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) to investigate both H2O2-mediated and H2O2-free NOR processes, corroborating the findings from the HG-FTIR analysis (Supplementary Fig. 26). Through these procedures, we affirmed the occurrence of the NOR with an elevated level of precision.
a, Common pollutants ordered by increasing nitrogen valence states. b, In situ DEMS is employed for qualitative and quantitative monitoring of nitrogen species within a sealed system, with a flow rate set at 0.8 ml min−1. ITO, indium tin oxide film. Please refer to Supplementary Fig. 28 for details. c, In situ HG-FTIR is utilized for the qualitative and quantitative analysis of nitrogen species within a hermetically sealed system, with a controlled flow rate set at 1 ml min−1. d, In situ electrochemical EPR is employed to precisely and quantitatively monitor alterations in NO levels throughout the NOR process within a sealed system.
EPR spectroscopy enabled us to notably enhance the detection limit to an absolute minimum spin number of 1.0 × 109, equivalent to femtomole-level quantification. Furthermore, NO, serving as the sole marker, can be captured by N-methyl-d-glucamine dithiocarbamate (MGD) to generate a distinctive signal25,26. Hence, we employed the same three-step approach outlined earlier to investigate the occurrence of NOR. Upon introducing the gas into our closed system, the NO signal instantaneously emerges (Fig. 2d). Under a constant potential of 1.2 V, after 30 min of N2O oxidation, the NO signal stabilizes without further increase. Subsequently, under the influence of a higher potential of 2.8 V, the NO signal progressively amplifies, surpassing the initial concentration. EPR spectroscopy confirms the oxidation of N2 into NO. Please note that, although a small amount of NH3 is present in the nitrogen source, it does not oxidize to produce N2O, NO or other nitrogen oxide compounds (Supplementary Fig. 23)24,27. Instead, it undergoes direct oxidation to N2, as confirmed by both in situ DEMS and HG-FTIR spectroscopy (Fig. 2c). This is evident as no enhancement of nitrogen oxide signals occurs while the NH3 signal diminishes (Fig. 2c). This evidence bolsters our confidence in the authentic activation of N2 through oxidation reaction. In light of these findings, we successfully traversed the entire process of substantiating N2 oxidation activation, encompassing the identification and elimination of contaminants, as well as the generation of NO markers at elevated potentials. The presence of false positives has been negated throughout this comprehensive undertaking.
Electrochemical performance and mechanism analysis
We then initiated testing of electrochemical performance. Before these tests, we conducted a meticulous quantitative analysis of gas impurities, thus mitigating their impact (production rate of nitrogen pollutants, 0.12 nmol s−1 cm−2; Supplementary Fig. 27). Our initial tests were conducted in normal mode, wherein we employed high potential to facilitate the NOR. We conducted 2 h of steady-state potential testing at various potential levels until reaching a mass transfer-limited current plateau at 3.0 V (Fig. 3a). Over a wide potential range, the FE for NOR reached a peak of 9.3% at 3.0 V, with the highest \({{{\rm{NO}}}_{3}}^{-}\) yield recorded at 5.42 nmol s−1 cm−2 at 3.0 V (see Fig. 3b and Supplementary Figs. 29–33 for repeatability experiments, and Supplementary Fig. 8 for post-reaction material characterization). We continued to increase the voltage range to explore a broader spectrum of FE and yield. Beyond 3.2 V, both the FE and yield exhibited substantial declines, indicative of intense competition reactions.
a, The fitted constant-pressure curve for NOR initiation begins at 1.8 V in 0.1 M K2SO4, with each constant-pressure period lasting 2 h. In this context, j represents the current density (mA cm−2). b, FE and \({{{\rm{NO}}}_{3}}^{-}\) yield derived from a. The error bars represent the s.d. calculated from four independent measurements, and the mean value (centre point) is presented together with s.d. (bar height). c, In situ electrochemical EPR monitors the generation of oxygen radicals during the NOR process. The electrolyte consists of 0.1 M K2SO4, with 100 mM DMPO serving as an indicator for ·OH. d, Constant-pressure curve in 0.1 M K2SO4 with 100 ppm H2O2. e, FE and \({{{\rm{NO}}}_{3}}^{-}\) yield of NOR derived from d. The error bars represent the s.d. calculated from four independent measurements, and the mean value (centre point) is presented together with s.d. (bar height). f, In situ DEMS monitors the oxygen source during NOR at varying potentials in the presence of H2O2. g, In an aqueous environment containing 100 mM DMPO and introduced H2O2, resulting in the abundant generation of ·OH, we explored whether these ·OH species can spontaneously oxidize N2 to NO without the application of potential.
Additionally, we investigated the impact of H2O2 concentration and pH on the efficiency of NOR (Supplementary Figs. 34 and 35) to determine the optimal reaction efficiency and the best utilization of H2O2. We also included blank experiments under Ar gas conditions (Supplementary Fig. 36). Next, we employed in situ EPR spectroscopy to investigate the formation and ultimate destination of oxygen intermediates throughout the reaction, utilizing 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a trapping agent for oxygen free radicals. Our observations indicated the appearance of superoxide anion radicals (O2·−) at 2 V, probably serving as pivotal intermediates in the two-electron pathway of water oxidation to H2O2 (refs. 28,29,30). As we continued to escalate the potential, signals attributed to ·OH emerged at 2.4 V. Furthermore, with increasing potential, the signal intensity of O2·− gradually waned, while the signal strength of ·OH progressively intensified (Fig. 3c). Consequently, our electrochemical test results corroborated that the NOR commences at 2.4 V, seemingly closely associated with the generation of ·OH. Therefore, we ventured to introduce H2O2 to generate enough ·OH to mediate the NOR, potentially leading to a substantial enhancement in NOR efficiency.
Following the introduction of H2O2, we observed a substantial reduction in the overpotential of NOR, accompanied by a notable increase in the maximum FE, which reached 25.6% with 8.3 nmol s−1 cm−2 at 1.9 V (Fig. 3d,e). This represents a remarkable improvement, being 2.77 times for FE and 1.86 times for yield greater than that observed without the introduction of H2O2. To elucidate the oxygen source responsible for the \({{{\rm{NO}}}_{3}}^{-}\) generated in the NOR subsequent to the introduction of H2O2, we incorporated 18O-labelled H2O2 (ref. 18) into the reaction and utilized in situ DEMS to analyse the origin of the oxygen within the product. As depicted in Fig. 3f, it becomes evident that nearly all oxygen originates from H2O2 (ref. 18) rather than H2O (ref. 16), regardless of whether at low or high potentials (Supplementary Fig. 37). This supports our previous hypothesis that the involvement of H2O2 effectively circumvents the water oxidation to oxygen evolution, furnishing an oxygen source for NOR. It should be noted that, although the introduction of H2O2 improves the efficiency of NOR, it cannot avoid the occurrence of OER. Moreover, the exceptionally high oxidative potential of ·OH also greatly benefits the NOR. Naturally, a question arises: can ·OH catalyse NOR activity thermodynamically without the need for applied potential? To address this question, we employed in situ EPR spectroscopy to investigate N2 activity following the introduction of H2O2. In situations where H2O2 generated a substantial quantity of ·OH, we did not observe the production of NO (Fig. 3g), indicating a potential alternative pathway for nitrate production.
Expansion of catalyst scope
To broaden the applicability of the H2O2-mediated N2 activation method across catalysts, we opted for ten commonly used catalysts, each commercially available, to streamline experiments and ensure reproducibility, namely TiO2 (ref. 31), CrO2, Mn3O4, Fe3O4, Co3O4, Ru, Rh, Pd, Ir and Pt (Fig. 4). Across these catalysts, our observations showed that the H2O2 mediation method notably enhances NOR performance, boosting FE (three to seven times) while reducing the potential (0.2–1.6 V). Importantly, during the electrochemical oxidation process, most catalysts undergo varying degrees of oxidation, forming oxides, hydroxides and hydroxylated species, which serve as active sites driving the NOR. Moreover, concerning catalyst design principles, we advocate three pivotal factors: (1) having peroxidase-like activity to catalyse the generation of ·OH from H2O2 (generally, most metal oxide catalysts exhibit peroxidase-like activity, albeit with varying degrees of strength); (2) preferring catalysts with reduced OER activity to mitigate competitiveness in NOR (the OER is a parasitic reaction on all metal oxides and a grand challenge to circumvent); (3) selecting a catalyst with weaker adsorption of oxygen intermediates (*O and *OH) but strong N2 adsorption. A less stable oxygen-binding catalyst, offering a more reactive *O/*OH, leads to a lower energy barrier for cleaving the metal–O bond during the activation of N2.
Classic catalysts commonly used were assessed for their performance in H2O2-mediated NOR, considering FE, \({{{\rm{NO}}}_{3}}^{-}\) yield (bottom graph), and onset potential (top graph). Except for catalysts hindered by poor conductivity (TiO2 and CrO2) and competitive OER (Ru and Ir), the NOR performance of other catalysts notably improved across all three evaluation criteria following the H2O2-mediated process. The experimental conditions entailed a H2O2 concentration of 100 ppm in 0.1 M K2SO4, under which the obtained FE and potentials were optimized. The error bars represent the s.d. calculated from four independent measurements, and the mean value (centre point) is presented together with s.d. (bar height).
Techno-economic assessment and feasibility validation
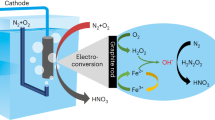
Building upon the established efficiency of H2O2 as a mediator for NOR to \({{{\rm{NO}}}_{3}}^{-}\), we proceeded to evaluate the economic viability of this approach. The existing thermochemical industry process for \({{{\rm{NO}}}_{3}}^{-}\) synthesis relies on raw materials such as CH4, H2O, O2 and N2, involving NH3 synthesis via the Haber–Bosch process, followed by NH3 oxidation to produce HNO3 through the Oswald process (Fig. 5a). This multi-step, high-temperature, high-pressure and energy-intensive method emits approximately 4.6 tonnes of CO2 for every tonne of HNO3 produced32,33. Leveraging our prior experience with the two-electron pathway for O2 reduction to generate H2O2 and established electrolytic cell design expertise34,35, we developed a flow cell for NOR to yield \({{{\rm{NO}}}_{3}}^{-}\) (Fig. 5a). In this design approach, the only required raw material is air. The cathode facilitates an O2 reduction via a two-electron pathway, generating H2O2. Subsequently, the electrolyte, rich in H2O2 and saturated with N2, flows to the anode to undergo NOR, yielding \({{{\rm{NO}}}_{3}}^{-}\). This streamlined process offers promising environmental and economic advantages compared with traditional methods. During a 190 h steady-state current test, we observed that an electrolytic cell voltage of approximately 1.69 V was necessary to achieve a current density of 15 mA cm−2, estimating a nitrate yield of 5.84 nmol s−1 cm−2 (Fig. 5b). Subsequently, we conducted an economic feasibility analysis, considering profit margins for electrocatalytic NOR to \({{{\rm{NO}}}_{3}}^{-}\) at a consistent current density from 0 to 100 mA cm−2, factoring in prevailing renewable energy electricity prices and devices (refer to Supplementary Information for detailed economic analysis formulae). As illustrated in Fig. 5c, profitability under direct use of commercial electricity presents formidable challenges, requiring a FE exceeding 90% at a current density approaching 100 mA cm−2, a goal that currently poses difficulties for the catalysis field. However, by harnessing solar panels to access renewable electricity, profitability becomes achievable with current catalytic capabilities (at 1.29 V, 15 mA cm−2, with an FE of 22%, the minimum profit is $0.4 per kilogram of HNO3). Additionally, to illustrate the diverse economic advantages of renewable energy, employing Pd/TiO2 as an anodic photocatalyst capable of driving the electrolytic cell cycle under light conditions presents an ideal approach for air-based nitrate production28, but the current efficiency of production remains relatively low. In essence, the utilization of renewable electricity stands as an inevitable solution for future sustainability, offering a pragmatic avenue to transform industrial processes related to NH3 and HNO3 production. Nonetheless, achieving industrial-scale production capabilities and efficiency demands considerable time and effort.
a, Comparison of production procedures and pros and cons of traditional thermochemical processes and advanced electrochemical flow cells. P and T represent pressure and temperature, respectively, with units of atmosphere and kelvins. b, The NOR and production of \({{{\rm{NO}}}_{3}}^{-}\) were assessed within an electrochemical flow cell setup. c, Assessment of the cost of electrocatalytic NOR for \({{{\rm{NO}}}_{3}}^{-}\) production.
Conclusion
In summary, we introduce an efficient approach for NOR by replacing the competitive OER with ·OH generated through H2O2 decomposition, serving as an active oxygen source. This provides a promising, sustainable alternative to the traditional, energy-intensive Haber–Bosch and Ostwald processes for direct nitrate synthesis from nitrogen. Through the design of an electrolytic cell that combines cathodic O2 reduction with anodic N2 oxidation, we demonstrate the viability of economically favourable \({{{\rm{NO}}}_{3}}^{-}\) production from air using renewable electricity. Furthermore, this concept of introducing active oxygen sources can be extended to other electrocatalytic oxidation reactions, including but not limited to methane and urea oxidation reactions, as well as organic small-molecule value-added oxidation processes, potentially revolutionizing the landscape of oxidation reaction pathways for sustainable production of value-added products.
Methods
Sample preparation
To prepare Pd/TiO2, we began by cutting titanium plates (TP) into small pieces (2.5 × 4.5 cm) and sonicating them in acetone, ethanol and distilled water for 30 min each. These pieces were then placed in a 50 ml Teflon-lined autoclave containing 40 ml of 5 M NaOH aqueous solution and the autoclave was kept at 180 °C for 24 h. After cooling to room temperature, the samples were washed multiple times with de-ionized (DI) water and ethanol, dried at 60 °C for 2 h. The samples were then immersed in 0.25 M Pd(NO3)2 solution for 1 h to exchange Na+ ions with Pd2+. The resulting PdTi2O5·H2O/TP was rinsed with DI water and ethanol multiple times and dried at 60 °C for 2 h. The samples were then annealed in a tube furnace at 500 °C under an Ar atmosphere for 2 h. After cooling to room temperature, the final product, Pd/TiO2/TP, was obtained. The only difference for the preparation of Ru, Rh, Ir, Pt and TiO2/TP is to soak the sample in 1 m HCl for 1 h to exchange Na+ with Ru3+, Rh3+, Ir3+, Pt2+ and H+. At last, two titanium sheets (2 × 4 cm) loaded with Pd/TiO2 were placed in 10 ml of ethanol and peeled off with an ultrasonic cell disruptor for 1 h. Subsequently, the obtained dispersion was centrifuged at 8,000 rpm for 10 min, and the precipitate containing Pd/TiO2 nanosheets was thoroughly washed with DI water and ethanol. We obtained CrO2, Mn3O4, Fe3O4 and Co3O4 from Aladdin Ltd.
Characterizations
X-ray diffraction patterns were acquired using a Bruker D8 Focus Diffraction System, employing a Cu Kα source (λ = 0.154178 nm, 40 kV, 30 mA). X-ray photoelectron spectroscopy spectra were gathered utilizing a Thermo Fisher Scientific K-Alpha+ instrument with monochromatic Al Kα radiation (hv = 1,486.6 eV). All spectra were obtained under a vacuum pressure of 2 × 10−7 Pa, and each sample underwent 20 cumulative scans. Binding energies were referenced to the C 1s peak at 284.6 eV. Scanning electron microscopy images were obtained using a Quanta FEG 250 field-emission scanning electron microscope. High-angle annular dark-field scanning transmission electron microscopy images and the corresponding electron energy-loss spectroscopy were collected using a JEOL JEM-ARM200F TEM/STEM instrument equipped with a spherical aberration corrector, operated at 200 kV. The concentration of produced nitrate was systematically detected and quantified using ion chromatography on a 930 Compact IC Flex instrument from Metrohm. FTIR spectroscopy was conducted using a Thermo Fisher Nicolet iS50 FTIR spectrometer, and when utilizing the MGD detector, liquid nitrogen was added. Gas-phase infrared spectra were acquired employing a cell cover with a 2 m light path. Raman spectra were obtained using a HORIBA LabRAM Odyssey with excitation by 532 nm laser light at a power of 10 mW. Absorbance data from the spectrophotometer were obtained using a SHIMADZU UV-1800 UV–vis spectrophotometer. EPR spectral signals were collected using Bruker A300 equipment. Mass spectrometry was performed using the HALO 201 instrument from Hiden Analytical.
In situ electrochemical Raman spectra test
Raman measurements were conducted in situ using the Horiba-Xplora Plus confocal microscope with a 532 nm laser (×50 objective, 10% filter, power range of 1–20 mW). In situ spectroelectrochemistry was conducted using a CHI760E electrochemical workstation in a membrane-separated flow cell, a Pt counter electrode from Alfa Aesar and a Hg/HgO reference electrode in a solution of 0.1 M N2-saturated K2SO4 with 100 ppm H2O2, with a volume of approximately 20 ml. The electrode sheet has a surface area of approximately 1 cm × 1.5 cm, with a catalyst loading of 100 µg cm−2. Before collecting each Raman spectrum signal, a 10 min pre-activation process is necessary to achieve a stable current density.
In situ ATR-SEIRAS measurements
In situ ATR-SEIRAS measurements were taken on a Nicolet iS50 FTIR spectrophotometer, a diamond-like carbon was coated onto a Si wafer (5 × 8 × 1 mm3) to prepare the internal reflection element (IRE). The coated IRE was ultrasonicated for 2 min with 30 wt.% concentrated H2SO4 followed by rinsing with DI water before experiments. Fifty microlitres of 2 mg ml−1 catalyst ink (no Nafion binder) was dropcast on the IRE and dried under air at room temperature. A glassy carbon paper was placed on top of the catalyst layer for good electrical contact. Glassy carbon rod connected to the IRE, Pt gauze and Ag/AgCl in 3 M KCl were used as the working electrode, counter electrode and reference electrode, respectively. An FTIR spectrometer with a mercury cadmium telluride detector was used for the in situ ATR-FTIR measurements. Gamry Reference 600 potentiostat is employed during recording of the infrared spectra.
Online DEMS measurement
DEMS measurements were conducted using a homemade electrochemical cell36. A solution of 0.1 M N2-saturated K2SO4 with or without 100 ppm H2O2 was continuously flowed into the cell through a peristaltic pump. Ar or N2 gas was bubbled constantly into the electrolyte before and during the DEMS measurements. A Ti mesh electrode coated with Pd/TiO2 catalyst (loading: 50 µg cm−2), a Pt wire and an Ag/AgCl electrode were used as the working electrode, counter electrode and reference electrode, respectively. The experimental setup included a breathable and hydrophobic membrane. Measurements were taken at potential of interest after the baseline was stable. Corresponding mass signals were recorded. After the electrochemical test was completed and the mass signal returned to baseline, the next cycle was started using the same conditions to minimize errors. The experiment was ended after several cycles. Throughout the testing process, the liquid inlet and liquid outlet are interlinked, establishing a closed electrocatalytic environment. As the electrochemical process unfolds, real-time analysis of the diversity of generated products proves advantageous. Within this recirculation system, the electrolyte volume amounts to approximately 30 ml with a flow rate of 18–20 ml h−1. We employed an external standard mass spectrometry method, wherein the concentration of the test product is determined by establishing a standard curve using the signal intensity of a known concentration standard (refer to Supplementary Fig. 21).
In situ HG-FTIR measurements
In situ HG-FTIR measurements were conducted using a Thermo Fisher Nicolet iS50 FTIR spectrophotometer. Gas-phase infrared spectroscopy and in-situ DEMS share a common set of electrolytic cells. In this setup, small gas molecules dissolved in the electrolyte are directed into the instrument for analysis within a vacuum environment, facilitated by a hydrophobic gas-permeable membrane. The gas flow rate is maintained at 1 ml min−1. Throughout the testing process, the liquid inlet and liquid outlet are interlinked, establishing a closed electrocatalytic environment. As the electrochemical process unfolds, real-time analysis of the diversity of generated products proves advantageous. Within this recirculation system, the electrolyte volume amounts to approximately 30 ml with a flow rate of 18–20 ml h−1. The quantification of nitrogen-containing species, including their content and types, can be determined by analysing the position and integrating the peak area of the infrared spectrum (refer to Supplementary Fig. 24 and Supplementary Table 1).
In situ electrochemistry EPR tests
In situ EPR experiments were performed using a Bruker A300 spectrometer operating at X-band frequency of 9.6 GHz and room temperature. The schematic diagram of the device is shown in Supplementary Fig. 28 (refs. 37,38). To prevent saturation of the EPR signal, the microwave power supplied to the resonator was set to 4 mW. The magnetic field modulation depth of 100 kHz was set to 0.1 mT or less to avoid distortion due to over-modulation. The conversion time and time constant were set to 40.96 ms and 20.48 ms, respectively. A commonly used rule of thumb is that the thickness of the liquid sample should be less than one-tenth of the wavelength of the microwave radiation used in the EPR experiment. This will minimize any absorption or scattering of the microwave radiation by the sample. A rubber cap is employed to hermetically seal the electrolytic cell, which contains 6 ml of electrolyte. Before the test, a 30 min infusion of N2 gas is conducted to guarantee saturation of the N2 gas within the electrolyte. When conducting tests involving ·OH and O2·−, a molar concentration of DMPO approximately 20–50 mM is used. During usage, it is common to add 0.1 mL of prepared DMPO to 0.9 ml of the target solution, resulting in an actual detection system equivalent of DMPO in the range of ~2–5 mM. For measurements involving O2·−, a higher DMPO concentration of about 100 mM is used. In the case of testing NO radicals, the trapping agent employed is MGD (Fe2+–MGD2) to capture NO formation. Fresh stock solutions of Fe–MGD (1:5) are prepared by adding ferrous sulfate to aqueous solutions of MGD.
Electrochemical measurements
For electrochemical NOR measurements, a CHI 760E electrochemical workstation was employed. A typical three-electrode H-cell configuration was used, consisting of a working electrode, an Ag/AgCl electrode (saturated KCl solution) as the reference electrode, and a Pt mesh counter electrode. Experiments were conducted in N2-saturated 0.1 M K2SO4 with or without 100 ppm H2O2 at a scan rate of 20 mV s−1. The H-cell was divided into a cathode cell (25 ml) and an anode cell (25 ml) by a Nafion membrane. Notably, iR correction was not applied, except under specific instructions.
For performance evaluation tests, electrochemical measurements were carried out using a CHI 760E electrochemical workstation with rotating disc electrode system. A glassy carbon rotation disk electrode (Φ = 3.0 mm) and specific electrodes (Ag/AgCl and platinum foil) were used. The rotating disc electrode was prepared by polishing with 1 μm alumina aqueous suspension for 5 minutes, followed by 0.05 μm alumina aqueous suspension for another 5 min, and subsequently ultrasonicated in DI water for 30 seconds. A homogeneous ink with a concentration of 4 mg ml−1 was prepared by dissolving 5 mg of Pd/TiO2 in a mixture of isopropanol and water (4:1 by volume) after 1 h of sonication. Twenty microlitres of ink were evenly spread onto the disk electrode surface and allowed to wet before entering the electrolyte (load capacity 100 μg cm−2). The electrocatalytic NOR process was initiated by bubbling N2 gas through the electrolyte at a flow rate of 20 ml min−1 for 2 h, while monitoring the potential against the RHE. Before NOR, linear sweep voltammetry was conducted until the polarization curves reached a steady state, using a scan rate of 20 mV s−1 within the voltage range of 1–2 V. NOR performance was assessed by linear sweep voltammetry in N2-saturated 0.1 M K2SO4 with or without 100 ppm H2O2 at a scan rate of 20 mV s−1 and a rotation speed of 1,600 rpm (refs. 34,39,40).
For the two-chamber electrolytic cell chronoamperometry test, a Ti piece (1 × 1 cm2) decorated with 100 μg of Pd/TiO2 was employed as the working electrode. Current density was normalized on the basis of the catalyst mass and the geometric electrode area. All electrochemical data were recorded in triplicate, with error bars representing the standard deviation (s.d.) of the measurements. All potentials were calibrated to the RHE using the equation ERHE = EAg/AgCl + 0.0591 × pH + φreference, where EAg/AgCl represents the experimental applied potential. It is worth noting that the correction term (0.0591 pH + φreference) was calibrated on the basis of a hydrogen reversible reaction.
For the NOR, the yield of \({{{\rm{NO}}}_{3}}^{-}\) was calculated using
The FE was calculated using
where \({{c}_{{\rm{NO}}{3}}}^{-}\) is the concentration of NOx−, V is the volume of the electrolyte in the anode compartment, t is the electrolysis time, m is the mass of the used catalyst, F is the Faradaic constant (96,485 C mol−1) and Q is the total charge passing the electrode.
Strict and standardized procedures were employed during the NOR tests to guarantee the accuracy of the data21. To ensure the reliability of the data, several procedures were followed such as avoiding introduction of nitrogen-containing compounds during material synthesis, ensuring the prepared electrolyte was sealed to exclude air, purifying the high-purity Ar/N2 gas before bubbling through the electrolyte to remove trace amounts of nitrogenous contaminants, cleaning any nitrogenous species trapped on Nafion membranes and maintaining the electrolytic cell in a relatively sealed state and prohibiting the entrance of nitrogen-containing compounds into the test room. Additional steps were taken to ensure the accuracy of the data: the Nafion membrane was cleaned by treating it in a 5% H2O2 solution at 80 °C for 1 h, followed by treatment in 5% dilute sulfuric acid at the same temperature for 1 h, and then in ultrapure water for another hour. The membrane was then sonicated in 0.05 M H2SO4 and ultrapure water for 15 min before use. New Nafion membrane was used every half a month and only used in NOR experiment to avoid any potential contamination. The high-purity N2 and Ar gases used were also pre-purified using NaOH, H2SO4, Na2SO4 and a silica gel sorbent tube to remove any potential Nitrogen contamination sources before the NOR test.
Quantification of H2O2
To quantify the generated H2O2, samples were collected at specific time intervals and combined with an equal volume of titanium oxysulfate solution (6 g l−1). The H2O2 yield was determined using the titanium oxysulfate indicator. The resulting complex solution was analysed using a UV–vis spectrophotometer at the maximum absorption wavelength λ = 406 nm.
H2O2 utilization efficiency
In a flow cell, the utilization efficiency of H2O2 over a specific time interval can be calculated as
where \(n{{{\rm{NO}}}_{3}}^{-}\), nH2O2 and n′H2O2 indicate produced \({{{\rm{NO}}}_{3}}^{-}\) and H2O2 and remaining H2O2 (every hour; μmol h−1), respectively.
Theoretical methods
All spin-polarized computations were carried out by the revised Perdewe–Burke–Ernzerhof functional41 based on DFT implemented in the Vienna ab initio Simulation Package (VASP)42,43. The plane-wave basis set with a cut-off energy of 450 eV was employed. The convergence thresholds of the total energy and the Hellmann–Feynman force are 10−4 eV and 0.03 eV Å−1, respectively. The van der Waals correction proposed by Grimme (DFT + D3) was chosen44. The search for the energy barrier and transition state along the minimum energy pathway was computed using the climbing-image nudged elastic band method45, for which five to nine intermediate images were interpolated between the initial and final states.
According to the experimental observations, both face-centred cubic Pd and rutile PdO2 have been considered for exploring the possible active sites and N2 oxidation mechanisms. For PdO2, the (110) surface has been investigated, which was modelled by a (2 × 3) supercell. Such supercell contains 12 atomic layers, for which the bottom 6 atomic layers were fixed to mimic the bulk, while the remaining atoms and adsorbates allowed to relax (Supplementary Fig. 10a). The Pd(111) surface was modelled by the supercell comprising of a five-layer slab with (4 × 4) lateral replication in the x–y plane, for which the three bottom atomic layers were fixed at their bulk positions (Supplementary Fig. 10b). For Pd(111) and PdO2(110) surfaces, the vacuum layer of larger than 15 Å was used to avoid the interactions between periodic images. Moreover, for Pd(111) and PdO2(110) surfaces, the Monkhorst–Pack meshes of 3 × 3 × 1 and 3 × 2 × 1 were adopted for structural optimization, respectively46.
For the electrochemical N2 oxidation reaction, the free energy change (∆G) for each elementary reaction step was calculated on the basis of the CHE model16, according to the equation ∆G = ∆E + ∆EZPE − T∆S, where ∆E is the reaction energy from DFT calculations, and ∆EZPE and T∆S (T = 298.15 K) are the contributions of the zero-point energy and entropy to ∆G, respectively. EZPE and TS for the free molecules were taken from the National Institutes of Standards Technology database47, and those of the adsorbed species (Supplementary Table 2) were obtained on the basis of the calculated vibrational frequencies dealt with the VASPKIT code48. The continuum solvent model as implemented in VASPsol code has been adopted to account for the solvation effect49, as verified in previous works18,19.
Data availability
The data supporting the finding of the study are available in the paper and its Supplementary Information. Source data are provided with this paper and in the Mendeley Data repository at https://doi.org/10.17632/kcwgbv68y6.1 (ref. 50).
References
Markit, I. Nitric Acid-Chemical Economics Handbook (IHS Markit, 2015).
Chen, J. G. et al. Beyond fossil fuel–driven nitrogen transformations. Science 360, eaar6611 (2018).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Galloway, J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Li, L. et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. 60, 14131–14137 (2021).
Wang, Y., Li, T., Yu, Y. & Zhang, B. Electrochemical synthesis of nitric acid from nitrogen oxidation. Angew. Chem. Int. Ed. 61, e202115409 (2022).
Iriawan, H. et al. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Primers 1, 56 (2021).
Nie, Z. et al. Catalytic kinetics regulation for enhanced electrochemical nitrogen oxidation by Ru-nanoclusters-coupled Mn3O4 catalysts decorated with atomically dispersed Ru atoms. Adv. Mater. 34, 2108180 (2022).
Kuang, M. et al. Efficient nitrate synthesis via ambient nitrogen oxidation with Ru-doped TiO2/RuO2 electrocatalysts. Adv. Mater. 32, 2002189 (2020).
Armstrong, D. A. et al. Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 87, 1139–1150 (2015).
Jin, H. et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 14, 354 (2023).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Grdeń, M., Łukaszewski, M., Jerkiewicz, G. & Czerwiński, A. Electrochemical behaviour of palladium electrode: oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 53, 7583–7598 (2008).
Plauck, A., Stangland, E. E., Dumesic, J. A. & Mavrikakis, M. Active sites and mechanisms for H2O2 decomposition over Pd catalysts. Proc. Natl Acad. Sci. USA 113, E1973–E1982 (2016).
Li, J., Staykov, A., Ishihara, T. & Yoshizawa, K. Theoretical study of the decomposition and hydrogenation of H2O2 on Pd and Au@Pd surfaces: understanding toward high selectivity of H2O2 synthesis. J. Phys. Chem. C 115, 7392–7398 (2011).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Ignarro, L. J., Fukuto, J. M., Griscavage, J. M., Rogers, N. E. & Byrns, R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proc. Natl Acad. Sci. USA 90, 8103–8107 (1993).
Anand, M., Abraham, C. S. & Nørskov, J. K. Electrochemical oxidation of molecular nitrogen to nitric acid-towards a molecular level understanding of the challenges. Chem. Sci. 12, 6442–6448 (2021).
Wan, H., Bagger, A. & Rossmeisl, J. Limitations of electrochemical nitrogen oxidation toward nitrate. J. Phys. Chem. Lett. 13, 8928–8934 (2022).
Guo, Y. et al. Electrochemical nitrate production via nitrogen oxidation with atomically dispersed Fe on N-doped carbon nanosheets. ACS Nano 16, 655–663 (2022).
A checklist for reproducibility in electrochemical nitrogen fixation. Nat. Commun. 13, 4642 (2022).
Choi, J. et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nat. Commun. 11, 5546 (2020).
Lee, S. A., Lee, M. G. & Jang, H. W. Catalysts for electrochemical ammonia oxidation: trend, challenge, and promise. Sci. China Mater. 65, 3334–3352 (2022).
Liu, H. Y. et al. Electrochemical ammonia oxidation with molecular catalysts. ACS Catal. 13, 4675–4682 (2023).
Xia, Y. & Zweier, J. L. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl Acad. Sci. USA 94, 12705–12710 (1997).
Bordignon, E. EPR spectroscopy of nitroxide spin probes. eMagRes 6, 235–254 (2017).
Li, Y. et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation. ACS Catal. 10, 3945–3957 (2020).
Dong, K. et al. Plasma-induced defective TiO2-x with oxygen vacancies: a high-active and robust bifunctional catalyst toward H2O2 electrosynthesis. Chem Catal. 1, 1437–1448 (2021).
Zhang, X. et al. Photothermal-assisted photocatalytic nitrogen oxidation to nitric acid on palladium-decorated titanium oxide. Adv. Energy Mater. 12, 2103740 (2022).
Hu, X. et al. Simultaneous generation of H2O2 and formate by co-electrolysis of water and CO2 over bifunctional Zn/SnO2 nanodots. Angew. Chem. Int. Ed. 62, e202304050 (2023).
Yu, M. et al. Self-supported Mo-doped TiO2 electrode for ambient electrocatalytic nitrogen oxidation. Electrochim. Acta 435, 141333 (2022).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
Lim, J., Fernández, C. A., Lee, S. W. & Hatzell, M. C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6, 3676–3685 (2021).
Dong, K. et al. Epoxidation of olefins enabled by an electro-organic system. Green Chem. 24, 8264–8269 (2022).
Dong, K. et al. Noble-metal-free electrocatalysts toward H2O2 production. J. Mater. Chem. A 8, 23123–23141 (2020).
Chen, W., He, F. & Chen, Y. X. in Encyclopedia of Solid–Liquid Interfaces, 497–513 (Elsevier, 2023).
Abdiaziz, K., Salvadori, E., Sokol, K. P., Reisner, E. & Roessler, M. M. Protein film electrochemical EPR spectroscopy as a technique to investigate redox reactions in biomolecules. Chem. Commun. 55, 8840–8843 (2019).
Chen, L. et al. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 221, 118747 (2022).
Dong, K. et al. Conductive two-dimensional magnesium metal–organic frameworks for high-efficiency O2 electroreduction to H2O2. ACS Catal. 12, 6092–6099 (2022).
Dong, K. et al. Honeycomb carbon nanofibers: a superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. 60, 10583–10587 (2021).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Monkhorst, H. J. & Pack, J. D. Special points for Brillonin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
NIST Standard Reference Database Number 69. National Institute of Standards and Technology http://webbook.nist.gov/chemistry/ (2023).
Wang, V., Xu, N., Liu, J. C., Tang, G. & Geng, W. T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Dong, K. H2O2-mediated electrosynthesis of nitrate from air. Mendeley Data https://doi.org/10.17632/kcwgbv68y6.1 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 22072015 and 21927811), the Free Exploration Project of Frontier Technology for Laoshan Laboratory (no. 16-02), the Program for Science & Technology Innovation Talents in Universities of Henan Province (no. 20HASTIT028) and China Postdoctoral Science Foundation (2023M731175 and GZB20230232). We thank S. Li from Nanjing University of Aeronautics and Astronautics and J. Nie from Jilin University for their invaluable contributions in the initial phases of the experiment. Furthermore, we appreciate X. Wang from Gaossunion Corporation for designing the in situ electrochemical electrolytic cell.
Author information
Authors and Affiliations
Contributions
K.D. performed the catalyst preparation, characterizations and catalytic tests. D.M. and Haobo Li conceived and conducted the theoretical investigation of the nitrogen oxidation mechanism. K.D., Y.Y., Huangjingwei Li, S.S., Y.W., Y.L., D.Z. and Qian Liu contributed to the structure characterizations and data analysis. K.D., Quan Li, D.M., X.S. and B.T. designed this study and wrote the paper. All authors contributed and reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Discussion, Technical economic analysis and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, K., Yao, Y., Li, H. et al. H2O2-mediated electrosynthesis of nitrate from air. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00522-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00522-8