Abstract
Nanoparticles exhibit anisotropy when distinct features can be identified along different axes. Such disruption in shape and/or composition symmetry can change how nanoparticles behave and interact with the surrounding environment compared with their isotropic counterparts. Anisotropic combinations can be limitless and show potential for tackling biological barriers and developing programmable, targeted, and combined delivery of bioactive molecules, mainly when featuring autonomous motion. In this Review, we summarize the main methods for the generation of anisotropic particles at the nanoscale. We further discuss how geometric cues or the incorporation of propulsive agents (chemically or physically driven) improve transport across biological fluids, promote cellular adhesion and internalization, and/or increase tissue penetration. We finally highlight considerations for the design of anisotropic nanoparticles and the precise control over morphology and properties, in addition to the challenges for clinical translation.
Key points
-
Anisotropic systems exhibit specific spatial-dependent properties based on shape, chemical composition and/or physical responsiveness.
-
Precise engineering of anisotropic nanoparticles remains challenging and could benefit from the introduction of biocompatible materials.
-
Introducing a virtually unlimited combination array of anisotropic cues into nanoparticle design expands their applicability for combined drug delivery, targeting and theranostics.
-
Methodologies to assess nanoparticle–biological environment interactions and transport require further standardization for successful clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Talebian, S. et al. Facts and figures on materials science and nanotechnology progress and investment. ACS Nano 15, 15940–15952 (2021).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
Pearce, A. K., Wilks, T. R., Arno, M. C. & O’Reilly, R. K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 5, 21–45 (2020).
Wadhwa, N. & Berg, H. C. Bacterial motility: machinery and mechanisms. Nat. Rev. Microbiol. 20, 161–173 (2022).
Constantino, M. A., Jabbarzadeh, M., Fu, H. C. & Bansil, R. Helical and rod-shaped bacteria swim in helical trajectories with little additional propulsion from helical shape. Sci. Adv. 2, e1601661 (2016).
Mesarec, L. et al. Normal red blood cells’ shape stabilized by membrane’s in-plane ordering. Sci. Rep. 9, 19742 (2019).
Iino, R., Kinbara, K. & Bryant, Z. Introduction: molecular motors. Chem. Rev. 120, 1–4 (2020).
Erickson, R. P., Jia, Z., Gross, S. P. & Yu, C. C. How molecular motors are arranged on a cargo is important for vesicular transport. PLoS Comput. Biol. 7, e1002032 (2011).
Luque, A., Zandi, R. & Reguera, D. Optimal architectures of elongated viruses. Proc. Natl Acad. Sci. USA 107, 5323–5328 (2010).
Welsch, S. et al. Electron tomography reveals the steps in filovirus budding. PLoS Pathog. 6, e1000875 (2010).
Zhang, Q. et al. Entry dynamics of single Ebola virus revealed by force tracing. ACS Nano 14, 7046–7054 (2020).
Choi, H., Jeong, S. H., Kim, T. Y., Yi, J. & Hahn, S. K. Bioinspired urease-powered micromotor as an active oral drug delivery carrier in stomach. Bioact. Mater. 9, 54–62 (2022).
Walker, D., Käsdorf, B. T., Jeong, H. H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Lin, R., Yu, W., Chen, X. & Gao, H. Self-propelled micro/nanomotors for tumor targeting delivery and therapy. Adv. Healthc. Mater. 10, e2001212 (2021).
Adriani, G. et al. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials 33, 5504–5513 (2012).
Thome, C. P., Hoertdoerfer, W. S., Bendorf, J. R., Lee, J. G. & Shields, C. W. Electrokinetic active particles for motion-based biomolecule detection. Nano Lett. 23, 2379–2387 (2023).
Ifra, Thodikayil, A. T. & Saha, S. Compositionally anisotropic colloidal surfactant decorated with dual metallic nanoparticles as a pickering emulsion stabilizer and their application in catalysis. ACS Appl. Mater. Interfaces 14, 23436–23451 (2022).
Glotzer, S. C. & Solomon, M. J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 6, 557–562 (2007).
Hu, S.-H. & Gao, X. Nanocomposites with spatially separated functionalities for combined imaging and magnetolytic therapy. J. Am. Chem. Soc. 132, 7234–7237 (2010).
Decuzzi, P. et al. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 141, 320–327 (2010).
Zhang, L. et al. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 181, 113–125 (2018).
Winkler, J. S., Barai, M. & Tomassone, M. S. Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds. Exp. Biol. Med. 244, 1162–1177 (2019).
Jiao, M., Li, W., Yu, Y. & Yu, Y. Anisotropic presentation of ligands on cargos modulates degradative function of phagosomes. Biophys. Rep. 2, 100041 (2022).
Shaghaghi, B., Khoee, S. & Bonakdar, S. Preparation of multifunctional Janus nanoparticles on the basis of SPIONs as targeted drug delivery system. Int. J. Pharm. 559, 1–12 (2019).
Wang, Z. et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv. Sci. 6, 1901690 (2019).
Niikura, K. et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 7, 3926–3938 (2013).
Zhang, W., Choi, H., Yu, B. & Kim, D.-H. Synthesis of iron oxide nanocube patched Janus magnetic nanocarriers for cancer therapeutic applications. Chem. Comm. 56, 8810–8813 (2020).
Zhang, M. et al. Precise synthesis of unique polydopamine/mesoporous calcium phosphate hollow Janus nanoparticles for imaging-guided chemo-photothermal synergistic therapy. Chem. Sci. 8, 8067–8077 (2017).
Rossi, F., Khoo, E. H., Su, X. & Thanh, N. T. K. Study of the effect of anisotropic gold nanoparticles on plasmonic coupling with a photosensitizer for antimicrobial film. ACS Appl. Bio Mater. 3, 315–326 (2020).
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A. & Danquah, M. K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018).
Harmsen, S. et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl Med. 7, 271ra277 (2015).
Tian, Y. et al. Gold nanostars functionalized with amine-terminated PEG for X-ray/CT imaging and photothermal therapy. J. Mater. Chem. B 3, 4330–4337 (2015).
Huang, X., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
Soleimany, A., Khoee, S., Dias, S. & Sarmento, B. Exploring low-power single-pulsed laser-triggered two-photon photodynamic/photothermal combination therapy using a gold nanostar/graphene quantum dot nanohybrid. ACS Appl. Mater. Interfaces 15, 20811–20821 (2023).
Li, Z. et al. Ce6-conjugated and polydopamine-coated gold nanostars with enhanced photoacoustic imaging and photothermal/photodynamic therapy to inhibit lung metastasis of breast cancer. Nanoscale 12, 22173–22184 (2020).
Rolland, J. P. et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 127, 10096–10100 (2005).
Hasan, W. et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 12, 287–292 (2012).
Galloway, A. L. et al. Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine 9, 523–531 (2013).
Glangchai, L. C., Caldorera-Moore, M., Shi, L. & Roy, K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J. Control. Release 125, 263–272 (2008).
Zhang, X. et al. Controllable subtractive nanoimprint lithography for precisely fabricating paclitaxel-loaded PLGA nanocylinders to enhance anticancer efficacy. ACS Appl. Mater. Interfaces 12, 14797–14805 (2020).
Esteban-Fernández de Ávila, B. et al. Nanomotor-enabled pH-responsive intracellular delivery of caspase-3: toward rapid cell apoptosis. ACS Nano 11, 5367–5374 (2017).
Ruiz-Gómez, S., Fernández-González, C. & Perez, L. Electrodeposition as a tool for nanostructuring magnetic materials. Micromachines 13, 1223 (2022).
Kang, C. & Honciuc, A. Self-assembly of Janus nanoparticles into transformable suprastructures. J. Phys. Chem. Lett. 9, 1415–1421 (2018).
Li, Z., Kesselman, E., Talmon, Y., Hillmyer, M. A. & Lodge, T. P. Multicompartment micelles from ABC miktoarm stars in water. Science 306, 98–101 (2004).
Khoee, S. & Nouri, A. in Design and Development of New Nanocarriers (ed. Grumezescu, A. M.) 145–180 (Elsevier, 2018).
Cui, H., Chen, Z., Zhong, S., Wooley, K. L. & Pochan, D. J. Block copolymer assembly via kinetic control. Science 317, 647–650 (2007).
Liu, X. et al. Multicompartment micelles based on hierarchical co-assembly of PCL-b-PEG and PCL-b-P4VP diblock copolymers. RSC Adv. 6, 5312–5319 (2016).
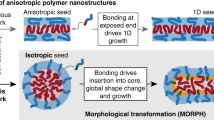
Hua, Z. et al. Anisotropic polymer nanoparticles with controlled dimensions from the morphological transformation of isotropic seeds. Nat. Commun. 10, 5406 (2019).
Penfold, N. J. W., Yeow, J., Boyer, C. & Armes, S. P. Emerging trends in polymerization-induced self-assembly. ACS Macro Lett. 8, 1029–1054 (2019).
Karagoz, B. et al. Polymerization-induced self-assembly (PISA) – control over the morphology of nanoparticles for drug delivery applications. Polym. Chem. 5, 350–355 (2014).
Li, Z. et al. Glyco-platelets with controlled morphologies via crystallization-driven self-assembly and their shape-dependent interplay with macrophages. ACS Macro Lett. 8, 596–602 (2019).
Inam, M. et al. 1D vs. 2D shape selectivity in the crystallization-driven self-assembly of polylactide block copolymers. Chem. Sci. 8, 4223–4230 (2017).
Zhang, J. et al. Shape memory actuation of Janus nanoparticles with amphipathic cross-linked network. ACS Macro Lett. 5, 1317–1321 (2016).
Yan, B. et al. Investigating switchable nanostructures in shape memory process for amphipathic Janus nanoparticles. ACS Appl. Mater. Interfaces 10, 36249–36258 (2018).
Keller, S., Toebes, B. J. & Wilson, D. A. Active, autonomous, and adaptive polymeric particles for biomedical applications. Biomacromolecules 20, 1135–1145 (2018).
Sun, J., Mathesh, M., Li, W. & Wilson, D. A. Enzyme-powered nanomotors with controlled size for biomedical applications. ACS Nano 13, 10191–10200 (2019).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA 104, 11901–11904 (2007).
Ben-Akiva, E. et al. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 6, eaay9035 (2020).
Florez, L. et al. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 8, 2222–2230 (2012).
Sun, Z. et al. Self-propelled Janus nanocatalytic robots guided by magnetic resonance imaging for enhanced tumor penetration and therapy. J. Am. Chem. Soc. 145, 11019–11032 (2023).
Chen, Z. et al. Enzyme-powered Janus nanomotors launched from intratumoral depots to address drug delivery barriers. Chem. Eng. J. 375, 122109 (2019).
Delcea, M. et al. Anisotropic multicompartment micro- and nano-capsules produced via embedding into biocompatible PLL/HA films. Chem. Comm. 47, 2098–2100 (2011).
Tang, S. et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 5, eaba6137 (2020).
Kloberg, M. J. et al. Surface-anisotropic Janus silicon quantum dots via masking on 2D silicon nanosheets. Adv. Mater. 33, e2100288 (2021).
Yang, Q., de Vries, M. H., Picchioni, F. & Loos, K. A novel method of preparing metallic Janus silica particles using supercritical carbon dioxide. Nanoscale 5, 10420–10427 (2013).
Yang, Q., Miao, X. & Loos, K. Fabrication of nano-sized hybrid Janus particles from strawberry-like hierarchical composites. Macromol. Chem. Phys. 219, 1800267 (2018).
Mani, K. A., Yaakov, N., Itzhaik Alkotzer, Y., Zelikman, E. & Mechrez, G. A robust fabrication method for amphiphilic Janus particles via immobilization on polycarbonate microspheres. Polymers 10, 900 (2018).
Kalashnikova, I., Bizot, H., Bertoncini, P., Cathala, B. & Capron, I. Cellulosic nanorods of various aspect ratios for oil in water pickering emulsions. Soft Matter 9, 952–959 (2013).
Hunter, S. J. & Armes, S. P. Pickering emulsifiers based on block copolymer nanoparticles prepared by polymerization-induced self-assembly. Langmuir 36, 15463–15484 (2020).
Jiang, S. & Granick, S. Controlling the geometry (Janus balance) of amphiphilic colloidal particles. Langmuir 24, 2438–2445 (2007).
Zhao, Z., Wang, W., Xiao, J., Chen, Y. & Cao, Y. Interfacial engineering of pickering emulsion co-stabilized by zein nanoparticles and Tween 20: effects of the particle size on the interfacial concentration of gallic acid and the oxidative stability. Nanomaterials 10, 1068 (2020).
Robin, B. et al. Tuning morphology of Pickering emulsions stabilised by biodegradable PLGA nanoparticles: how PLGA characteristics influence emulsion properties. J. Colloid Interface Sci. 595, 202–211 (2021).
Cai, S. et al. pH-responsive superstructures prepared via the assembly of Fe3O4 amphipathic Janus nanoparticles. Regen. Biomater. 5, 251–259 (2018).
Kadam, R., Ghawali, J., Waespy, M., Maas, M. & Rezwan, K. Janus nanoparticles designed for extended cell surface attachment. Nanoscale 12, 18938–18949 (2020).
Wang, J., Jansen, J. A. & Yang, F. Electrospraying: possibilities and challenges of engineering carriers for biomedical applications-a mini review. Front. Chem. 7, 258 (2019).
Sanchez-Vazquez, B., Amaral, A. J. R., Yu, D. G., Pasparakis, G. & Williams, G. R. Electrosprayed Janus particles for combined photo-chemotherapy. AAPS PharmSciTech 18, 1460–1468 (2017).
Li, K. et al. Enhanced fluorescent intensity of magnetic-fluorescent bifunctional PLGA microspheres based on Janus electrospraying for bioapplication. Sci. Rep. 8, 17117 (2018).
Hwang, S. et al. Anisotropic hybrid particles based on electrohydrodynamic co-jetting of nanoparticle suspensions. Phys. Chem. Chem. Phys. 12, 11894–11899 (2010).
Roh, K. H., Martin, D. C. & Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 4, 759–763 (2005).
Gregory, J. V. et al. Programmable delivery of synergistic cancer drug combinations using bicompartmental nanoparticles. Adv. Healthc. Mater. 9, e2000564 (2020).
Ge, K. et al. Gold nanorods with spatial separation of CeO2 deposition for plasmonic-enhanced antioxidant stress and photothermal therapy of Alzheimer’s disease. ACS Appl. Mater. Interfaces 14, 3662–3674 (2022).
Ye, J. et al. Quantitative photoacoustic diagnosis and precise treatment of inflammation in vivo using activatable theranostic nanoprobe. Adv. Funct. Mater. 30, 2001771 (2020).
Li, Q. et al. Nanosized Janus AuNR-Pt motor for enhancing NIR-II photoacoustic imaging of deep tumor and Pt2+ ion-based chemotherapy. ACS Nano 16, 7947–7960 (2022).
Li, R. et al. In situ production of Ag/polymer asymmetric nanoparticles via a powerful light-driven technique. J. Am. Chem. Soc. 141, 19542–19545 (2019).
Ji, X. et al. Multifunctional parachute-like nanomotors for enhanced skin penetration and synergistic antifungal therapy. ACS Nano 15, 14218–14228 (2021).
Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Simultaneous two drugs release form Janus particles prepared via polymerization-induced phase separation approach. Colloids Surf. B 170, 85–91 (2018).
Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Fabricating cauliflower-like and dumbbell-like Janus particles: loading and simultaneous release of DOX and ibuprofen. Colloids Surf. B 173, 155–163 (2019).
Dehghani, E., Barzgari-Mazgar, T., Salami-Kalajahi, M. & Kahaie-Khosrowshahi, A. A pH-controlled approach to fabricate electrolyte/non-electrolyte Janus particles with low cytotoxicity as carriers of DOX. Mater. Chem. Phys 249, 123000 (2020).
Li, Y. et al. Morphology evolution of Janus dumbbell nanoparticles in seeded emulsion polymerization. J. Colloid Interface Sci. 543, 34–42 (2019).
Vatankhah, Z., Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Seed’s morphology-induced core-shell composite particles by seeded emulsion polymerization for drug delivery. Colloids Surf. B 191, 111008 (2020).
Meyer, R. A. & Green, J. J. Shaping the future of nanomedicine: anisotropy in polymeric nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8, 191–207 (2016).
Zhang, L., Chen, Q., Ma, Y. & Sun, J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 3, 107–120 (2019).
Hasani-Sadrabadi, M. M. et al. Morphological tuning of polymeric nanoparticles via microfluidic platform for fuel cell applications. J. Am. Chem. Soc. 134, 18904–18907 (2012).
Angly, J. et al. Microfluidic-induced growth and shape-up of three-dimensional extended arrays of densely packed nanoparticles. ACS Nano 7, 6465–6477 (2013).
Sun, X. T. et al. Microfluidic preparation of polymer-lipid Janus microparticles with staged drug release property. J. Colloid Interface Sci. 553, 631–638 (2019).
Xie, H., She, Z.-G., Wang, S., Sharma, G. & Smith, J. W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 28, 4459–4463 (2012).
Hao, N., Nie, Y., Tadimety, A., Closson, A. B. & Zhang, J. X. J. Microfluidics-mediated self-template synthesis of anisotropic hollow ellipsoidal mesoporous silica nanomaterials. Mater. Res. Lett. 5, 584–590 (2017).
Palagi, S. & Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 3, 113–124 (2018).
Zhang, Y. & Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 5, 500–510 (2021).
Saper, G. & Hess, H. Synthetic systems powered by biological molecular motors. Chem. Rev. 120, 288–309 (2020).
Soong, R. K. et al. Powering an inorganic nanodevice with a biomolecular motor. Science 290, 1555–1558 (2000).
Tu, Y., Peng, F. & Wilson, D. A. Motion manipulation of micro‐ and nanomotors. Adv. Mater. 29, 1701970 (2017).
Peng, F., Tu, Y. & Wilson, D. A. Micro/nanomotors towards in vivo application: cell, tissue and biofluid. Chem. Soc. Rev. 46, 5289–5310 (2017).
Li, N. et al. Chemotactic NO/H2S nanomotors realizing cardiac targeting of G-CSF against myocardial ischemia-reperfusion injury. ACS Nano 17, 12573–12593 (2023).
Peng, F., Tu, Y., van Hest, J. C. & Wilson, D. A. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew. Chem. Int. Ed. 54, 11662–11665 (2015).
Liu, X. et al. Enzyme-powered hollow nanorobots for active microsampling enabled by thermoresponsive polymer gating. ACS Nano 16, 10354–10363 (2022).
Wu, Z. et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 4, eaat4388 (2018).
Schattling, P. S., Ramos-Docampo, M. A., Salgueiriño, V. & Städler, B. Double-fueled Janus swimmers with magnetotactic behavior. ACS Nano 11, 3973–3983 (2017).
Abdelmohsen, L. K. et al. Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor. ACS Nano 10, 2652–2660 (2016).
Wang, L. et al. Continuous microfluidic self-assembly of hybrid Janus-like vesicular motors: autonomous propulsion and controlled release. Small 11, 3762–3767 (2015).
Archer, R. A. et al. pH‐responsive catalytic Janus motors with autonomous navigation and cargo‐release functions. Adv. Funct. Mater. 30, 2000324 (2020).
Díez, P. et al. Ultrafast directional Janus Pt-mesoporous silica nanomotors for smart drug delivery. ACS Nano 15, 4467–4480 (2021).
Wu, Z. et al. Water-powered cell-mimicking Janus micromotor. Adv. Funct. Mater. 25, 7497–7501 (2015).
Gao, W. et al. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9, 117–123 (2015).
Peng, F. et al. A peptide functionalized nanomotor as an efficient cell penetrating tool. Chem. Comm. 53, 1088–1091 (2017).
Tu, Y. et al. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 11, 1957–1963 (2017).
Pijpers, I. A. B. et al. Hybrid biodegradable nanomotors through compartmentalized synthesis. Nano Lett. 20, 4472–4480 (2020).
Nance, E. A. et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl Med. 4, 149ra119 (2012).
Ma, X., Hortelão, A. C., Patiño, T. & Sánchez, S. Enzyme catalysis to power micro/nanomachines. ACS Nano 10, 9111–9122 (2016).
Ma, X. et al. Enzyme-powered hollow mesoporous Janus nanomotors. Nano Lett. 15, 7043–7050 (2015).
Rucinskaite, G., Thompson, S. A., Paterson, S. & de la Rica, R. Enzyme-coated Janus nanoparticles that selectively bind cell receptors as a function of the concentration of glucose. Nanoscale 9, 5404–5407 (2017).
Toebes, B. J., Cao, F. & Wilson, D. A. Spatial control over catalyst positioning on biodegradable polymeric nanomotors. Nat. Commun. 10, 5308 (2019).
Wang, L., Hortelão, A. C., Huang, X. & Sánchez, S. Lipase-powered mesoporous silica nanomotors for triglyceride degradation. Angew. Chem. Int. Ed. 58, 7992–7996 (2019).
Fu, D., Ye, Y., Gao, C., Xie, D. & Peng, F. Bienzymatic spiky Janus nanomotors powered by histamine. ChemNanoMat 8, e202200152 (2022).
Hu, Y., Li, Z. & Sun, Y. Ultrasmall enzyme/light-powered nanomotor facilitates cholesterol detection. J. Colloid Interface Sci. 621, 341–351 (2022).
Patiño, T. et al. Influence of enzyme quantity and distribution on the self-propulsion of non-Janus urease-powered micromotors. J. Am. Chem. Soc. 140, 7896–7903 (2018).
Llopis-Lorente, A. et al. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. ACS Nano 13, 12171–12183 (2019).
Arque, X. et al. Autonomous treatment of bacterial infections in vivo using antimicrobial micro- and nanomotors. ACS Nano 16, 7547–7558 (2022).
Hortelão, A. C., Carrascosa, R., Murillo-Cremaes, N., Patiño, T. & Sánchez, S. Targeting 3D bladder cancer spheroids with urease-powered nanomotors. ACS Nano 13, 429–439 (2019).
Valles, M., Pujals, S., Albertazzi, L. & Sánchez, S. Enzyme purification improves the enzyme loading, self-propulsion, and endurance performance of micromotors. ACS Nano 16, 5615–5626 (2022).
Hao, L.-W. et al. Microfluidic-directed magnetic controlling supraballs with multi-responsive anisotropic photonic crystal structures. J. Mater. Sci. Technol. 81, 203–211 (2021).
Zhang, B. et al. Twin-bioengine self-adaptive micro/nanorobots using enzyme actuation and macrophage relay for gastrointestinal inflammation therapy. Sci. Adv. 9, eadc8978 (2023).
Wu, Z. et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew. Chem. Int. Ed. 52, 7000–7003 (2013).
Wan, M. et al. Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 10, 966 (2019).
Wu, Z. et al. Carrier-free trehalose-based nanomotors targeting macrophages in inflammatory plaque for treatment of atherosclerosis. ACS Nano 16, 3808–3820 (2022).
Li, J., Mayorga‐Martinez, C. C., Ohl, C. D. & Pumera, M. Ultrasonically propelled micro‐ and nanorobots. Adv. Funct. Mater. 32, 2102265 (2022).
Garcia-Gradilla, V. et al. Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications. ACS Nano 7, 9232–9240 (2013).
Garcia-Gradilla, V. et al. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small 10, 4154–4159 (2014).
Esteban-Fernández de Ávila, B. et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 10, 4997–5005 (2016).
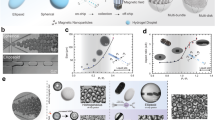
Cardoso, V. F. et al. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 7, 1700845 (2018).
Gao, W., Sattayasamitsathit, S., Manesh, K. M., Weihs, D. & Wang, J. Magnetically powered flexible metal nanowire motors. J. Am. Chem. Soc. 132, 14403–14405 (2010).
Schamel, D. et al. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano 8, 8794–8801 (2014).
Ghosh, A., Paria, D., Rangarajan, G. & Ghosh, A. Velocity fluctuations in helical propulsion: how small can a propeller be. J. Phys. Chem. Lett. 5, 62–68 (2014).
Venugopalan, P. L., Jain, S., Shivashankar, S. & Ghosh, A. Single coating of zinc ferrite renders magnetic nanomotors therapeutic and stable against agglomeration. Nanoscale 10, 2327–2332 (2018).
Venugopalan, P. L. et al. Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 14, 1968–1975 (2014).
Ramachandran, R. V. et al. How safe are magnetic nanomotors: from cells to animals. Bio. Adv. 140, 213048 (2022).
Shen, Y. et al. Adaptive control of nanomotor swarms for magnetic-field-programmed cancer cell destruction. ACS Nano 15, 20020–20031 (2021).
Xu, L., Mou, F., Gong, H., Luo, M. & Guan, J. Light-driven micro/nanomotors: from fundamentals to applications. Chem. Soc. Rev. 46, 6905–6926 (2017).
Shao, J. et al. Erythrocyte membrane modified Janus polymeric motors for thrombus therapy. ACS Nano 12, 4877–4885 (2018).
Peng, X. et al. Opto-thermoelectric microswimmers. Light Sci. Appl. 9, 141 (2020).
Sridhar, V. et al. Carbon nitride-based light-driven microswimmers with intrinsic photocharging ability. Proc. Natl Acad. Sci. USA 117, 24748–24756 (2020).
Cao, S. et al. Photoactivated nanomotors via aggregation induced emission for enhanced phototherapy. Nat. Commun. 12, 2077 (2021).
Chen, S. et al. Dual-source powered nanomotor with integrated functions for cancer photo-theranostics. Biomaterials 288, 121744 (2022).
Zheng, S. et al. Biocompatible nanomotors as active diagnostic imaging agents for enhanced magnetic resonance imaging of tumor tissues in vivo. Adv. Funct. Mater. 31, 2100936 (2021).
Liu, J. et al. Rotary biomolecular motor-powered supramolecular colloidal motor. Sci. Adv. 9, eabg3015 (2023).
Zhou, D. et al. Light-ultrasound driven collective “firework” behavior of nanomotors. Adv. Sci. 5, 1800122 (2018).
Li, J. et al. Magneto-acoustic hybrid nanomotor. Nano Lett. 15, 4814–4821 (2015).
Shao, J. et al. Twin-engine Janus supramolecular nanomotors with counterbalanced motion. J. Am. Chem. Soc. 144, 11246–11252 (2022).
Yu, M. et al. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett. 16, 7176–7182 (2016).
Guo, M. et al. Impacts of particle shapes on the oral delivery of drug nanocrystals: mucus permeation, transepithelial transport and bioavailability. J. Control. Release 307, 64–75 (2019).
Bao, C. et al. Enhanced transport of shape and rigidity-tuned α-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett. 20, 1352–1361 (2020).
Sosnik, A., das Neves, J. & Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog. Polym. Sci. 39, 2030–2075 (2014).
Wang, Z. H. et al. Self-thermophoretic nanoparticles enhance intestinal mucus penetration and reduce pathogenic bacteria interception in colorectal cancer. Adv. Funct. Mater. 33, 2212013 (2023).
Geng, Y. et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2, 249–255 (2007).
Xiong, F. et al. Superparamagnetic anisotropic nano-assemblies with longer blood circulation in vivo: a highly efficient drug delivery carrier for leukemia therapy. Nanoscale 8, 17085–17089 (2016).
Zhou, Z. et al. Linear-dendritic drug conjugates forming long circulating nanorods for cancer-drug delivery. Biomaterials 34, 5722–5735 (2013).
Kapate, N., Clegg, J. R. & Mitragotri, S. Non-spherical micro- and nanoparticles for drug delivery: progress over 15 years. Adv. Drug Deliv. Rev. 177, 113807 (2021).
van de Ven, A. L. et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Release 158, 148–155 (2012).
Jurney, P. et al. Unique size and shape-dependent uptake behaviors of non-spherical nanoparticles by endothelial cells due to a shearing flow. J. Control. Release 245, 170–176 (2017).
Cooley, M. et al. Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 10, 15350–15364 (2018).
Gupta, R., Badhe, Y., Mitragotri, S. & Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 12, 6318–6333 (2020).
Sikder, A., Pearce, A. K., Kumar, C. M. S. & O’Reilly, R. K. Elucidating the role of multivalency, shape, size and functional group density on antibacterial activity of diversified supramolecular nanostructures enabled by templated assembly. Mater. Horiz. 10, 171–178 (2023).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Barua, S. et al. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl Acad. Sci. USA 110, 3270–3275 (2013).
Wong, S. H. D. et al. Anisotropic nanoscale presentation of cell adhesion ligand enhances the recruitment of diverse integrins in adhesion structures and mechanosensing-dependent differentiation of stem cells. Adv. Funct. Mater. 29, 1806822 (2019).
Da Silva-Candal, A. et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Control. Release 309, 94–105 (2019).
Acter, S., Vidallon, M. L. P., Crawford, S., Tabor, R. F. & Teo, B. M. Bowl-shaped mesoporous polydopamine nanoparticles for size-dependent endocytosis into HeLa cells. ACS Appl. Nano Mater. 4, 9536–9546 (2021).
Acter, S., Vidallon, M. L. P., Crawford, S., Tabor, R. F. & Teo, B. M. Efficient cellular internalization and transport of bowl‐shaped polydopamine particles. Part. Part. Syst. Charact. 37, 2000166 (2020).
Talamini, L. et al. Influence of size and shape on the anatomical distribution of endotoxin-free gold nanoparticles. ACS Nano 11, 5519–5529 (2017).
García-Álvarez, R., Hadjidemetriou, M., Sánchez-Iglesias, A., Liz-Marzán, L. M. & Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 10, 1256–1264 (2018).
Joseph, A. et al. Chemotactic synthetic vesicles: design and applications in blood-brain barrier crossing. Sci. Adv. 3, e1700362 (2017).
Brown, T. D., Habibi, N., Wu, D., Lahann, J. & Mitragotri, S. Effect of nanoparticle composition, size, shape, and stiffness on penetration across the blood-brain barrier. ACS Biomater. Sci. Eng. 6, 4916–4928 (2020).
Kolhar, P. et al. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl Acad. Sci. USA 110, 10753–10758 (2013).
Nowak, M., Brown, T. D., Graham, A., Helgeson, M. E. & Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 5, e10153 (2020).
Han, S. et al. Spatiotemporal tracking of gold nanorods after intranasal administration for brain targeting. J. Control. Release 357, 606–619 (2023).
Ju, Y. et al. Monodisperse Au-Fe2C Janus nanoparticles: an attractive multifunctional material for triple-modal imaging-guided tumor photothermal therapy. ACS Nano 11, 9239–9248 (2017).
Deng, Q. et al. Biological mediator-propelled nanosweeper for nonpharmaceutical thrombus therapy. ACS Nano 15, 6604–6613 (2021).
Xie, S. et al. Self-propelling nanomotors integrated with biofilm microenvironment-activated NO release to accelerate healing of bacteria-infected diabetic wounds. Adv. Healthc. Mater. 11, e2201323 (2022).
Wibroe, P. P. et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 12, 589–594 (2017).
Kumar, S., Anselmo, A. C., Banerjee, A., Zakrewsky, M. & Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 220, 141–148 (2015).
Shukla, S. et al. The impact of aspect ratio on the biodistribution and tumor homing of rigid soft-matter nanorods. Adv. Healthc. Mater. 4, 874–882 (2015).
Li, Z. et al. Shape effect of glyco-nanoparticles on macrophage cellular uptake and immune response. ACS Macro Lett. 5, 1059–1064 (2016).
Meyer, R. A. et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 11, 1519–1525 (2015).
Hassani Najafabadi, A. et al. Cancer immunotherapy via targeting cancer stem cells using vaccine nanodiscs. Nano Lett. 20, 7783–7792 (2020).
Kuai, R. et al. Robust anti-tumor T cell response with efficient intratumoral infiltration by nanodisc cancer immunotherapy. Adv. Ther. 3, 2000094 (2020).
Kadiyala, P. et al. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 13, 1365–1384 (2019).
Scheetz, L. et al. Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin. Cancer Res. 26, 4369–4380 (2020).
Tazaki, T. et al. Shape-dependent adjuvanticity of nanoparticle-conjugated RNA adjuvants for intranasal inactivated influenza vaccines. RSC Adv. 8, 16527–16536 (2018).
Wang, Z. et al. Fluidity-guided assembly of Au@Pt on liposomes as a catalase-powered nanomotor for effective cell uptake in cancer cells and plant leaves. ACS Nano 16, 9019–9030 (2022).
Ou, J. et al. MnO2-based nanomotors with active Fenton-like Mn2+ delivery for enhanced chemodynamic therapy. ACS Appl. Mater. Interfaces 13, 38050–38060 (2021).
Yang, Z. et al. Ultrasmall enzyme-powered Janus nanomotor working in blood circulation system. ACS Nano 17, 6023–6035 (2023).
Choi, H., Cho, S. H. & Hahn, S. K. Urease-powered polydopamine nanomotors for intravesical therapy of bladder diseases. ACS Nano 14, 6683–6692 (2020).
Tong, F. et al. Carbon monoxide-propelled nanomotors as an active treatment for renal injury. Appl. Mater. Today 32, 101823 (2023).
Kiristi, M. et al. Lysozyme-based antibacterial nanomotors. ACS Nano 9, 9252–9259 (2015).
Hansen-Bruhn, M. et al. Active intracellular delivery of a Cas9/sgRNA complex using ultrasound-propelled nanomotors. Angew. Chem. Int. Ed. 57, 2657–2661 (2018).
Wang, W. et al. Acoustic propulsion of nanorod motors inside living cells. Angew. Chem. Int. Ed. 53, 3201–3204 (2014).
Pal, M. et al. Maneuverability of magnetic nanomotors inside living cells. Adv. Mater. 30, e1800429 (2018).
Zhang, X. et al. NIR-propelled Janus nanomotors for active photoacoustic imaging and synergistic photothermal/chemodynamic therapy. J. Colloid Interface Sci. 648, 457–472 (2023).
Meng, J. et al. Pyroelectric Janus nanomotors to promote cell internalization and synergistic tumor therapy. J. Control. Release 357, 342–355 (2023).
Liu, Y. et al. NIR-II-activated yolk-shell nanostructures as an intelligent platform for Parkinsonian therapy. ACS Appl. Bio Mater. 3, 6876–6887 (2020).
Jarvis, M., Krishnan, V. & Mitragotri, S. Nanocrystals: a perspective on translational research and clinical studies. Bioeng. Transl. Med. 4, 5–16 (2019).
Jahn, M. R. et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 78, 480–491 (2011).
Takahashi, N., Higashi, K., Ueda, K., Yamamoto, K. & Moribe, K. Determination of nonspherical morphology of doxorubicin-loaded liposomes by atomic force microscopy. J. Pharm. Sci. 107, 717–726 (2018).
Ekanem, E. E., Zhang, Z. & Vladisavljevic, G. T. Facile production of biodegradable bipolymer patchy and patchy Janus particles with controlled morphology by microfluidic routes. Langmuir 33, 8476–8482 (2017).
Cao, X., Li, W., Ma, T. & Dong, H. One-step fabrication of polymeric hybrid particles with core–shell, patchy, patchy Janus and Janus architectures via a microfluidic-assisted phase separation process. RSC Adv. 5, 79969–79975 (2015).
Du, J. & O’Reilly, R. K. Anisotropic particles with patchy, multicompartment and Janus architectures: preparation and application. Chem. Soc. Rev. 40, 2402–2416 (2011).
Walther, A. & Muller, A. H. Janus particles: synthesis, self-assembly, physical properties, and applications. Chem. Rev. 113, 5194–5261 (2013).
Lesov, I. et al. Bottom-up synthesis of polymeric micro- and nanoparticles with regular anisotropic shapes. Macromolecules 51, 7456–7462 (2018).
Verhoef, J. J. F. et al. Iron nanomedicines induce Toll-like receptor activation, cytokine production and complement activation. Biomaterials 119, 68–77 (2017).
Scott, L. J. Ferric carboxymaltose: a review in iron deficiency. Drugs 78, 479–493 (2018).
Acknowledgements
H.A. and J.d.N. gratefully acknowledge Fundação para a Ciência e a Tecnologia, Portugal, for financial support (2020.06264.BD fellowship and CEECIND/01280/2018 contract under the Individual CEEC Program, respectively).
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conceptualization of the article, data search and discussion of content. H.A. wrote the initial draft of the manuscript. G.T., B.S. and J.d.N. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
Financial competing interests for G.T. that may be interpreted as related to the current manuscript include current and prior funding (Supplementary Tables 2 and 3) from Novo Nordisk, CSL Vifor, Hoffman La Roche, Oracle, Draper Laboratory, Massachusetts Institute of Technology (MIT) Lincoln Laboratory, National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering, National Cancer Institute, Advanced Research Projects Agency for Health), Bill and Melinda Gates Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, Karl van Tassel (1925) Career Development Professor, MIT and the Defense Advanced Research Projects Agency, as well as employment by the MIT and Brigham and Women’s Hospital (Supplementary Table 1). Personal financial interests include equity/stock (Lyndra Therapeutics, Suono Bio, Vivtex, Celero Systems, Syntis Bio), board of directors membership and/or consulting (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio) and royalties (past and potentially in the future) from licensed and/or optioned intellectual property (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio, Johns Hopkins, MIT, and Mass General Brigham Innovation). Complete details of all relationships for-profit and not-for-profit for G.T. can be found in the Supplementary Information. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Takuro Niidome and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almeida, H., Traverso, G., Sarmento, B. et al. Nanoscale anisotropy for biomedical applications. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00169-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00169-2