Featured
-
-
Article
| Open Access Enantioselective total synthesis of (‒)-lucidumone enabled by tandem prins cyclization/cycloetherification sequence
Enantioselective total synthesis of (‒)-lucidumone enabled by tandem prins cyclization/cycloetherification sequence
The Ganoderma meroterpenoids are a growing class of natural products with a wide range of biological activities. Here, the authors report the enantioselective total synthesis of the Ganoderma meroterpenoid (‒)-lucidumone, featuring a copper-catalyzed enantioselective silicon-tethered intramolecular Diels-Alder cycloaddition to assemble the bicyclo[2.2.2]octane framework and a domino deprotection/Prins reaction/cycloetherification/oxidation sequence to generate concurrenly the tetrahydrofuran and the fused indanone skeleton.
- Xian-Zhang Liao
- , Ran Wang
- & Guang Li
-
Article
| Open Access Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
While the homo N-N coupling of two NH moieties to form the hydrazide N-N bond is well developed, the crossdehydrogenative hetero N-N coupling remains unevolved. Here the authors present an efficient, PhI(OAc)2-mediated intermolecular N-N cross-coupling of primary benzamides with primary and secondary amines.
- Subban Kathiravan
- , Prakriti Dhillon
- & Ian A. Nicholls
-
Article
| Open Access Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Carbon isotope labelling of bioactive molecules is essential for accessing the pharmacokinetic and pharmacodynamic properties of new drug entities. Here, the authors propose an electrochemical isotope-labelling protocol which enables the use of near-stoichiometric 14CO2, facilitating late-stage and single-step carbon-14 labelling of pharmaceuticals and representative precursors.
- Gabriel M. F. Batista
- , Ruth Ebenbauer
- & Troels Skrydstrup
-
Article
| Open Access A concise and scalable chemoenzymatic synthesis of prostaglandins
A concise and scalable chemoenzymatic synthesis of prostaglandins
Prostaglandins are of interest to synthetic chemists due to their biological activities. Here, the authors present a concise chemoenzymatic synthesis method for several representative prostaglandins, achieved in 5 to 7 steps, via the common intermediate bromohydrin, a radical equivalent of Corey lactone.
- Yunpeng Yin
- , Jinxin Wang
- & Jian Li
-
Article
| Open Access Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
P-stereogenic heterocycles are privileged chiral ligands and bioactive compounds, but the catalytic asymmetric synthesis of P-stereogenic phosphindane derivatives is challenging. Here, the authors report a catalytic kinetic resolution of phosphindole oxides via rhodium-catalyzed diastereo- and enantioselective conjugate addition to access enantiopure Pstereogenic phosphindane and phosphindole derivatives.
- Long Yin
- , Jiajia Li
- & Dong Guo
-
Article
| Open Access Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Chemically modified nucleobases and oligonucleotides are essential in several fields but introducing functional groups into nucleobases requires laborious chemical synthesis. Here, the authors report site-selective alkylation at the C8-position of guanines in guanosine, GMP, GDP, and GTP, as well as late-stage alkylation of RNA/DNA oligonucleotides through photomediated Minisci reaction.
- Ruoqian Xie
- , Wanlu Li
- & Gang Chen
-
Article
| Open Access Targeted sampling of natural product space to identify bioactive natural product-like polyketide macrolides
Targeted sampling of natural product space to identify bioactive natural product-like polyketide macrolides
Polyketide macrolides are of interest for drug discovery but their inherent structural and stereochemical complexity hinders the exploration of related regions of chemical space more broadly. Here, the authors designed in silico and synthesized a library of tetrahydrofuran-containing polyketide macrolides, and screened them against a panel of biological assays, identifying biologically active library members.
- Darryl M. Wilson
- , Daniel J. Driedger
- & Robert A. Britton
-
Article
| Open Access Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Medium-sized-ring compounds are challenging synthetic targets in organic chemistry. Here the authors report an intramolecular dearomative [5 + 4] cycloaddition of naphthalene-derived vinylcyclopropanes to construct these molecules under visible-light irradiation and a proper triplet photosensitizer.
- Min Zhu
- , Yuan-Jun Gao
- & Shu-Li You
-
Article
| Open Access 2H-Thiopyran-2-thione sulfine, a compound for converting H2S to HSOH/H2S2 and increasing intracellular sulfane sulfur levels
2H-Thiopyran-2-thione sulfine, a compound for converting H2S to HSOH/H2S2 and increasing intracellular sulfane sulfur levels
Reactive sulfane sulfur species such as persulfides and H2S2 are important redox regulators and linked to H2S signaling, but their study is hindered by a lack of suitable donors to produce them. Here, the authors report 2H-thiopyran-2-thione sulfine (TTS), a compound which can specifically convert H2S to HSOH, and then to H2S2 in the presence of excess H2S.
- Qi Cui
- , Meg Shieh
- & Ming Xian
-
Article
| Open Access Synthesis of meta-carbonyl phenols and anilines
Synthesis of meta-carbonyl phenols and anilines
Phenols and anilines are of extreme importance for medicinal chemistry and material science but the selective preparation of meta-substituted phenols and anilines remains challenging. Here the authors report an efficient copper-catalyzed dehydrogenation strategy to exclusively synthesize meta-carbonyl phenols and anilines from carbonyl substituted cyclohexanes.
- Bao-Yin Zhao
- , Qiong Jia
- & Yong-Qiang Wang
-
Article
| Open Access N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
Planar-chiral cyclophanes are important for drug discovery but the enantioselective synthesis of planar-chiral cyclophanes remains challenging. Here the authors describe an N-heterocyclic carbene-catalyzed asymmetric construction of planar-chiral cyclophanes.
- Jiayan Li
- , Ziyang Dong
- & Changgui Zhao
-
Article
| Open Access Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Combining data science and organic synthesis to achieve the rapid and precise creation of complex molecules while controlling multiple selectivities is an emerging trend, but few successful examples are reported. Here, the authors develop an artificial neural network regression model using bond orbital data to predict chemical reactivities.
- Shingo Harada
- , Hiroki Takenaka
- & Tetsuhiro Nemoto
-
Article
| Open Access Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis
Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis
Characterizing highly-reactive glycosyl cation intermediates and understanding their glycosylation mechanisms are essential to the stereoselective synthesis of complex carbohydrates. Here the authors report a workflow that is utilized to characterize rhamnosyl 1,3-bridged dioxanium ions derived from C-3 p-anisoyl esterified donors.
- Peter H. Moons
- , Floor ter Braak
- & Thomas J. Boltje
-
Article
| Open Access Enantioselective functionalization of unactivated C(sp3)–H bonds through copper-catalyzed diyne cyclization by kinetic resolution
Enantioselective functionalization of unactivated C(sp3)–H bonds through copper-catalyzed diyne cyclization by kinetic resolution
Site- and stereoselective C–H functionalization is challenging in the synthetic chemistry, and the catalytic enantioselective C(sp3)–H functionalization based on vinyl cations has scarcely explored. Here, the authors report an asymmetric copper-catalyzed tandem diyne cyclization/unactivated C(sp3)–H insertion reaction via a kinetic resolution.
- Yang-Bo Chen
- , Li-Gao Liu
- & Long-Wu Ye
-
Article
| Open Access Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Stereodefined vinylboron compounds are important organic synthons, but the selective generation of the thermodynamically unfavorable Z-isomers remains challenging. Here, the authors develop a cobalt catalytic system, enabling the transformation of terminal alkynes with excellent Z-selectivity, even in the presence of bulky substituents.
- Jinglan Wen
- , Yahao Huang
- & Peng Hu
-
Article
| Open Access Identification of a potent palladium-aryldiphosphine catalytic system for high-performance carbonylation of alkenes
Identification of a potent palladium-aryldiphosphine catalytic system for high-performance carbonylation of alkenes
The development of stable and efficient ligands is of vital significance to enhance the catalytic performance of carbonylation reactions of alkenes. Here, the authors develop an aryldiphosphine ligand, used in palladium-catalyzed regioselective carbonylation of alkenes, exhibiting high catalytic performance and strong oxygen-resistance stability.
- Kang Zhao
- , Hongli Wang
- & Feng Shi
-
Article
| Open Access Silver-catalyzed direct conversion of epoxides into cyclopropanes using N-triftosylhydrazones
Silver-catalyzed direct conversion of epoxides into cyclopropanes using N-triftosylhydrazones
Epoxides are prominent small-ring O-heterocycles found in a variety of bioactive natural products and pharmaceuticals. Here, the authors report a silver carbene strategy to achieve O-to-C atom exchange of epoxides in a single step, affording diverse fluoroalkylcyclopropanes.
- Linxuan Li
- , Paramasivam Sivaguru
- & Xihe Bi
-
Article
| Open Access On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
On-surface synthesis relies on carefully designed molecular precursors that are thermally activated to afford desired, covalently coupled architectures. Here, the authors study the intramolecular reactions of vinyl groups in a poly-para-phenylene-based model system and provide a comprehensive description of the reaction steps taking place on the Au(111) surface under ultrahigh vacuum conditions.
- Marco Di Giovannantonio
- , Zijie Qiu
- & Roman Fasel
-
Article
| Open Access Dimethyl sulfate and diisopropyl sulfate as practical and versatile O-sulfation reagents
Dimethyl sulfate and diisopropyl sulfate as practical and versatile O-sulfation reagents
O-Sulfation is a vital post-translational modification in bioactive molecules, yet there are significant challenges for the synthesis. Here, the authors report a general and robust approach to O-sulfation by harnessing the tunable reactivity of dimethyl sulfate or diisopropyl sulfate under tetrabutylammonium bisulfate activation.
- Shuaishuai Yue
- , Guoping Ding
- & Jiakun Li
-
Article
| Open Access Dioxygen compatible electron donor-acceptor catalytic system and its enabled aerobic oxygenation
Dioxygen compatible electron donor-acceptor catalytic system and its enabled aerobic oxygenation
The photochemical properties of Electron Donor-Acceptor (EDA) complexes present exciting opportunities, but often require an inert atmosphere to maintain high efficiency. Here, the authors develop a photocatalytic system through rational design, which overcomes the oxygen-sensitive limitation of traditional EDA photocatalytic systems.
- Jialiang Wei
- , Junhong Meng
- & Ning Jiao
-
Article
| Open Access General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs
General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs
Many alkyl fluorides are readily available, but the synthetic applications trail behind the widely accepted utility of other halides. Here, the authors report a practical Csp3-F bond functionalization method that expands the currently restricted synthetic space of unactivated C(sp3)-F bonds, but also uses benzylic, propargylic and acyl fluorides.
- D. Lucas Kane
- , Bryan C. Figula
- & Christian Wolf
-
Article
| Open Access Zirconium and hafnium catalyzed C–C single bond hydroboration
Zirconium and hafnium catalyzed C–C single bond hydroboration
Selective cleavage and subsequent functionalization of C−C single bonds present a fundamental challenge, and the use of inexpensive and Earth-abundant group IV metals for catalytic C−C single-bond cleavage is largely underdeveloped. Here, the authors report zirconium-catalyzed C−C single-bond cleavage and subsequent hydroboration reactions.
- Sida Li
- , Haijun Jiao
- & Lipeng Wu
-
Article
| Open Access Nickel-catalyzed electrophiles-controlled enantioselective reductive arylative cyclization and enantiospecific reductive alkylative cyclization of 1,6-enynes
Nickel-catalyzed electrophiles-controlled enantioselective reductive arylative cyclization and enantiospecific reductive alkylative cyclization of 1,6-enynes
Transition metal-catalyzed asymmetric cyclization of 1,6-enynes is a powerful tool for the construction of chiral nitrogen-containing heterocycles, but limiting to the use of aryl or alkenyl metal reagents. Here, the authors report Ni-catalyzed enantioselective anti-arylative cyclization and enantiospecific cis-alkylative cyclization of 1,6-enynes.
- Wenfeng Liu
- , Yunxin Xing
- & Kun Shen
-
Article
| Open Access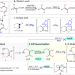 Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
The synthesis of highly substituted olefins has been pursued for decades. Here, the authors show a carbonyl-directed sequential Heck/isomerization/C–H arylation of alkenes for modular synthesis of stereodefined all-carbon tetrasubstituted olefins.
- Runze Luan
- , Ping Lin
- & Weiping Su
-
Article
| Open Access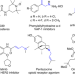 Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
The oxidative intermolecular amination of C-H bonds represents a straightforward method to construct aliphatic allylic amines. However, the utilization of widely available internal alkenes remains a synthetic challenge. Here, the authors present a regioselective Cu-catalyzed oxidative allylic C-H amination of internal olefins with azodiformates.
- Le Wang
- , Cheng-Long Wang
- & Shu-Yu Zhang
-
Article
| Open Access Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Enhancing the sp3-hybridized character of molecular structures in drug discovery is paramount. Here, the authors introduce a deoxygenative cross-electrophile coupling technique that pairs easily accessible carboxylic acid-derived redox-active esters with aldehyde sulfonyl hydrazones, employing Eosin Y as an organophotocatalyst under visible light irradiation.
- Stefano Bonciolini
- , Antonio Pulcinella
- & Timothy Noël
-
Article
| Open Access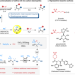 Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Stoichiometric amounts of metal reductant and the requirement of a directing group limit the application of transition metal-catalyzed reductive difunctionalization of alkenes with alkyl halides. Here, the authors report a reductive difunctionalization of alkenes via a radical-anion relay with Na2S2O4 as both reductant and sulfone-source.
- Xiaoya Hou
- , Hongchi Liu
- & Hanmin Huang
-
Review Article
| Open Access Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
α,α-Disubstituted α-amino acids (α-AAs) have improved properties compared to other amino acids. Here, the authors provide an overview of the recent advancements since 2015 and discuss existing challenges for their synthesis.
- Yu Zhang
- , Jaro Vanderghinste
- & Shoubhik Das
-
Article
| Open Access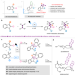 Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Conformationally rigid 3D molecules are of high interest. Here the authors develop intermolecular aza-Paternò-Büchi reactions to access ladder-shape azetidine-fused indoline pentacycles with excellent regiodivergency and stereoselectivity.
- Jianjian Huang
- , Tai-Ping Zhou
- & Fangrui Zhong
-
Article
| Open Access Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Photoexcitation of the electron-donor-acceptor (EDA) complexes are an effective approach to achieve radicals by triggering electron transfer but the catalytic version of EDA complex photoactivation remains underdeveloped. Here, the authors introduce 4-nitrophthalonitrile as an electron acceptor to facilitate π-stacking with electron-rich aromatics to form EDA complexes.
- Ting Xue
- , Cheng Ma
- & Rong Zeng
-
Article
| Open Access Cross-catenation between position-isomeric metallacages
Cross-catenation between position-isomeric metallacages
The study of cross-catenated metallacages could provide facile insights into achieving more precise control over low-symmetry/high-complexity hierarchical assembly systems but is currently lacking. Here, the authors report a cross-catenane formed between two position-isomeric Pt(II) metallacages in the solid state.
- Yiliang Wang
- , Taotao Liu
- & Jun Li
-
Article
| Open Access Water-stable boroxine structure with dynamic covalent bonds
Water-stable boroxine structure with dynamic covalent bonds
Despite the structural significance of boroxines in different classes of materials, their applicability in aqueous media is limited by their hydrolytic instability. Here, the authors discovered a water-stable boroxine structure with excellent pH stability and water-compatible dynamic covalent bonds.
- Xiaopei Li
- , Yongjie Zhang
- & Guangyan Qing
-
Article
| Open Access Carbene organic catalytic planar enantioselective macrolactonization
Carbene organic catalytic planar enantioselective macrolactonization
Macrolactones exhibit distinct conformational and configurational properties and are widely found in natural products but catalysts to govern both macrolactone formation and stereochemical control remain largely unexplored. Here, the authors disclose an N-heterocyclic carbene (NHC)-catalyzed enantioselective synthesis of chiral macrolactones varying in ring size from sixteen to twenty members that feature distinct configurationally stable planar stereogenicity.
- Xiaokang Lv
- , Fen Su
- & Yonggui Robin Chi
-
Article
| Open Access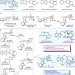 Collective total synthesis of stereoisomeric yohimbine alkaloids
Collective total synthesis of stereoisomeric yohimbine alkaloids
Nature generates the stereoisomeric yohimbine alkaloids using bioavailable monoterpene secolaganin as the building block. Here, the authors reset the stage by the development of a bioinspired coupling, in which the pentacyclic skeleton is constructed through enantioselective kinetic resolution of an achiral, easily accessible synthetic surrogate.
- Meiyi Tang
- , Haigen Lu
- & Liansuo Zu
-
Article
| Open Access Location-agnostic site-specific protein bioconjugation via Baylis Hillman adducts
Location-agnostic site-specific protein bioconjugation via Baylis Hillman adducts
Proteins labelled site-specifically with small molecules are valuable assets for chemical biology and drug development. Here, the authors report Baylis Hillman orchestrated protein aminothiol labelling (BHoPAL), a bioconjugation strategy for specific labelling of the 1,2-aminothiol moiety and combine it with a lipoic acid ligase-based technology to achieve labelling at any desired site within proteins.
- Mudassir H. Mir
- , Sangeeta Parmar
- & Dimpy Kalia
-
Article
| Open Access Mechanochemical synthesis of organoselenium compounds
Mechanochemical synthesis of organoselenium compounds
While the reported methods for the synthesis of organoselenium compounds mainly employ preformed activated selenium reagents, the direct utilization of elemental selenium in the synthesis is a more attractive but challenging strategy. Here, the authors report the rapid synthesis of versatile organoselenium compounds via mechanical stimulation.
- Shanshan Chen
- , Chunying Fan
- & Xiaofeng Wei
-
Article
| Open Access C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
Sulfur fluoride exchange (SuFEx), a new type of linkage reaction, has great potential for application in functional molecule linkage to prepare pharmaceuticals, biomolecules, and polymers. Herein, the authors report the synthesis of a range of sulfoximines through SuFEx reaction between sulfonimidoyl fluorides and aryl/alkyl organotrifluoroborates.
- Suqin Zhao
- , Daming Zeng
- & Xuefeng Jiang
-
Article
| Open Access Enantioselective synthesis of [4]helicenes by organocatalyzed intermolecular C-H amination
Enantioselective synthesis of [4]helicenes by organocatalyzed intermolecular C-H amination
Current methods to construct helical chiral molecules for asymmetric catalysis almost entirely rely on catalytic enantiocontrolled fused-ring system extension. Herein, the authors report a direct terminal peri-functionalization strategy, which allows for efficient assembling of substituted carbohelicenes via an organocatalyzed enantioselective amination reaction.
- Xihong Liu
- , Boyan Zhu
- & Rui Wang
-
Article
| Open Access Total syntheses of Tetrodotoxin and 9-epiTetrodotoxin
Total syntheses of Tetrodotoxin and 9-epiTetrodotoxin
Tetrodotoxin and congeners are specific voltage-gated sodium channel blockers that exhibit remarkable anesthetic and analgesic effects but total synthesis procedures are often limited by the scale. Here, the authors present a scalable asymmetric syntheses of Tetrodotoxin and 9-epiTetrodotoxin from the abundant chemical feedstock furfuryl alcohol.
- Peihao Chen
- , Jing Wang
- & Xiangbing Qi
-
Article
| Open Access Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Open-shell catalytically active species are widely used in energy-consuming redox reactions, but their excited-state lifetimes are usually short. Here, the authors report a closed-shell thioxanthone-hydrogen anion species generated under electrochemical conditions, which can be photochemically converted to a potent and long-lived reductant.
- Wen-Jie Kang
- , Yanbin Zhang
- & Hao Guo
-
Article
| Open Access Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation
Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation
Direct and site-selective C–H functionalization of alkenes under environmentally benign conditions represents a useful and attractive yet challenging transformation to access value-added molecules. Here, the authors report a protocol for a variety of intermolecular Heck-type functionalization of C(sp2)–H bond of alkenes by thianthrenation.
- Ming-Shang Liu
- , Hai-Wu Du
- & Wei Shu
-
Article
| Open Access Synthesis of inter-[60]fullerene conjugates with inherent chirality
Synthesis of inter-[60]fullerene conjugates with inherent chirality
Inter-fullerene conjugates are non-naturally occurring carbon allotropes. Here the authors report on the chemical synthesis and solid-state structure of three inter-[60]fullerene hybrids with inherent chirality.
- Yoshifumi Hashikawa
- , Shu Okamoto
- & Yasujiro Murata
-
Article
| Open Access Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles
Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles
N–N axially chiral motifs are of synthetic interest due to their presence in natural products, pharmaceuticals, and chiral ligands. Here, the authors develop a direct catalytic synthesis of N–N atropisomers with simultaneous creation of contiguous axial and central chirality by oxidative NHC catalyzed (3 + 3) cycloaddition.
- Shao-Jie Wang
- , Xia Wang
- & Shenci Lu
-
Article
| Open Access Regio- and enantioselective synthesis of acyclic quaternary carbons via organocatalytic addition of organoborates to (Z)-Enediketones
Regio- and enantioselective synthesis of acyclic quaternary carbons via organocatalytic addition of organoborates to (Z)-Enediketones
All-carbon quaternary stereocenters are an important synthetic motif but are especially difficult to synthesize enantioselectively. Here, the authors demonstrate the organocatalytic regio- and enantioselective synthesis of valuable acyclic 1,4-dicarbonyl products with vinylated and arylated quaternary centers.
- Po-Kai Peng
- , Andrew Isho
- & Jeremy A. May
-
Article
| Open Access Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Developing efficient methods for the formation and cleavage of amide species is a primary research goal, but the amide C–N bond cleavage is exceptionally challenging. Here, the authors report the development of an organocatalyst that can effectively catalyze the atroposelective ring-opening of biaryl lactams via amide C–N bond cleavage.
- Yuxing Cai
- , Yuxin Zhao
- & Yong Huang
-
Article
| Open Access C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
Bioisosteric design has become an essential approach during drug molecule discovery process. Here, the authors describe a strategy for defluorinative couplings of propellanes, enabling the synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres.
- Mingshuo Chen
- , Yuang Cui
- & Xiaheng Zhang
-
Article
| Open Access Unlocking regioselective meta-alkylation with epoxides and oxetanes via dynamic kinetic catalyst control
Unlocking regioselective meta-alkylation with epoxides and oxetanes via dynamic kinetic catalyst control
Regioselective arene C−H bond alkylation is a powerful tool in synthetic chemistry, yet subject to many challenges. Here, the authors report the meta-C−H bond alkylation of aromatics bearing N-directing groups using (hetero)aromatic epoxides as alkylating agents.
- Peng-Bo Bai
- , Alastair Durie
- & Igor Larrosa
-
Article
| Open Access Atomic-resolution structure analysis inside an adaptable porous framework
Atomic-resolution structure analysis inside an adaptable porous framework
Single crystal X-ray diffraction is one of the most powerful structure elucidation tools, but it’s challenging to determine complex structures. Here the authors report a metal-organic framework for encapsulation and immobilization of various guests using highly ordered internal water network, obtaining high quality atomic-resolution data.
- Yuki Wada
- , Pavel M. Usov
- & Masaki Kawano
-
Article
| Open Access Modular access to chiral bridged piperidine-γ-butyrolactones via catalytic asymmetric allylation/aza-Prins cyclization/lactonization sequences
Modular access to chiral bridged piperidine-γ-butyrolactones via catalytic asymmetric allylation/aza-Prins cyclization/lactonization sequences
N/O-Heterocycles are widely spread in natural products and drug candidates. Here, the authors report a modular access to bridged piperidine-γ-butyrolactones via Cu/Ir-catalyzed asymmetric allyation and aza-Prins cyclization/lactonization sequences.
- Cong Fu
- , Ling He
- & Chun-Jiang Wang

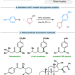 Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides
Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides