Abstract
Background
Type 2 diabetes mellitus (T2DM) is recognized an independent risk factor for chronic kidney disease (CKD). The precise contribution and differential response to treatment strategies to reduce kidney dysfunction, depending on whether obesity is present alongside T2DM or not, remain to be fully clarified. Our objective was to improve our understanding of how obesity contributes to kidney function in patients with T2DM and coronary heart disease (CHD), who are highly predisposed to CKD, to assign the most effective dietary approach to preserve kidney function.
Methods
1002 patients with CHD and estimated glomerular filtration rate (eGFR)≥30 ml/min/1.73m2, were randomized to consume a Mediterranean diet (35% fat, 22% MUFA, < 50% carbohydrates) or a low-fat diet (28% fat, 12% MUFA, > 55% carbohydrates). Patients were classified into four groups according to the presence of T2DM and/or obesity at baseline: Non-Obesity/Non-T2DM, Obesity/Non-T2DM, Non-Obesity/T2DM and Obesity/T2DM. We evaluated kidney function using serum creatinine-based estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (uACR) before and after 5-years of dietary intervention.
Results
Patients with Obesity/T2DM had the lowest baseline eGFR and the highest baseline uACR compared to non-diabetics (p < 0.05). After dietary intervention, the Mediterranean diet induced a lower eGFR decline in patients with Obesity/T2DM, compared to a low-fat diet but not in the other groups (p = 0.014). The Mediterranean diet, but not the low-fat diet, also reduced uACR only in patients with Obesity/T2DM (p = 0.024).
Conclusions
Obesity provided an additive effect to T2DM resulting in a more pronounced decline in kidney function compared to T2DM alone when compared to non-diabetics. In patients with concomitant presence of T2DM and obesity, with more metabolic complications, consumption of a Mediterranean diet seemed more beneficial than a low-fat diet in terms of preserving kidney function. These findings provide valuable insights for tailoring personalized lifestyle modifications in secondary prevention of cardiovascular disease.
Trial registration
URL, http://www.cordioprev.es/index.php/en. Clinicaltrials.gov number, NCT00924937

Similar content being viewed by others
Background
The prevalence of cardiometabolic conditions has risen due to different factors such as longer life expectancy, lifestyle modifications and improved diagnostic tools [1,2,3]. In fact, chronic kidney disease (CKD), which affects over 10% of the total adult population, is marked by a gradual decline of kidney function, leading to end-stage renal disease, and imposing significant costs on the healthcare system [4]. Type 2 diabetes (T2DM) is recognized as an independent risk factor for CKD, where persistent or uncontrolled hyperglycemia triggers various pathways contributing to kidney dysfunction, including excessive reactive oxygen species production, hypoxia, mitochondrial dysfunction and inflammation [5, 6]. However, it is not only T2DM but also obesity that contributes to kidney impairment [7], either indirectly through systemic arterial hypertension or directly through lipid accumulation in the glomerulus leading to structural changes such as increased permeability, glomerulosclerosis or albuminuria [8, 9]. Furthermore, obesity has been associated with reduced estimated glomerular filtration rate (eGFR), a comprehensive indicator of kidney function [10, 11]. Nonetheless, the impact of obesity whether accompanied by diabetes or not, on kidney dysfunction remains uncertain [12, 13]. As a result, this unclear relationship may lead to ineffective treatment approaches depending on whether obesity and T2DM coexist or are present separately.
Lifestyle modifications, particularly dietary changes, are proposed as primary strategies in the management of T2DM and obesity [14]. In this regard, different results from prospective observational studies and clinical trials have underscored the significance of individual nutrients, specific foods and overall dietary patterns in preventing T2DM and its associated comorbidities. It emphasizes the importance of the quality of dietary fats and carbohydrates consumed, rather than solely focusing on their quantity [15]. Diets characterized by a high intake of vegetables, fruit, whole grains, nuts, legumes, and low in red/processed meats, refined grains, and sugar-sweetened beverages have demonstrated efficacy in the reduction of the risk of developing T2DM and the improvement of glycemic control [16,17,18,19]. We have recently found that the long-term consumption of a healthy dietary pattern (a Mediterranean diet or a low-fat diet) reduced the risk of T2DM development in patients with coronary heart disease (CHD) [20]. Interestingly, in the context of this study, consumption of these diets also increased the probability of diabetes remission in patients with newly-diagnosed T2DM [21]. Furthermore, the Mediterranean diet, but not the low-fat diet, was associated with a slower decline in kidney function, as measured by eGFR, particularly in patients with T2DM [22].
Taking all the above factors into in account, this study aimed to more precisely assess the impact of obesity, in combination with T2DM, to kidney impairment in patients with CHD who are highly predisposed to CKD, in order to assign the most suitable dietary strategy (a Mediterranean diet and/or a low-fat diet) and thus further preserve kidney function.
Methods
Design and study population
This work was performed within the framework of the CORDIOPREV study (Clinicaltrials.gov number NCT00924937), a single center, prospective, randomized, single-blind and controlled dietary intervention clinical trial that includes 1002 patients with CHD. This study was developed at Reina Sofia University Hospital in Córdoba, Spain. The volunteers followed one of two different dietary models, a Mediterranean or a low-fat diet, for 7 years, in addition to their conventional treatment for CHD. Details of the rationale, study methods, inclusion and exclusion criteria, as well as a description of the cardiovascular risk factors and the patients’ baseline characteristics have been previously described [23]. Patients provided written informed consent to participate in the study. The trial protocol was approved by the ethics committee of Reina Sofia University Hospital in Cordoba (No. 1496/27/03/2009), following the principles of the Helsinki Declaration and good clinical practices.
In the CORDIOPREV study, the assessment of kidney function and the analysis of the impact of T2DM and obesity on kidney impairment were not defined before the start of the study.
Randomization and dietary intervention
Randomization was performed by the Andalusian School of Public Health. More details on the randomization procedures were previously described [23]. In addition to the conventional treatment for CHD, each patient was randomly assigned to follow one of these dietary patterns: (a) the Mediterranean diet, with a minimum of 35% of total calories from fat [22% monounsaturated fat (MUFA), 6% polyunsaturated fat (PUFA), and < 10% saturated fat (SFA)], 15% proteins, and a maximum of 50% carbohydrates and (b) a low-fat, high complex carbohydrate diet, as recommended by the National Cholesterol Education Program, with < 30% of total calories from fat (12–14% MUFAs, 6–8% PUFAs, < 10% SFAs), ≥ 55% from carbohydrates and 15% from protein. In both diets, the cholesterol content was adjusted to < 300 mg/day. While both study diets incorporated foods from all major food groups, no specific total calorie restriction was set. Comprehensive details on dietary assessment and follow-up visits have been published previously [23, 24]. The research included no interventions to increase physical activity or weight loss. Participants in both intervention groups were given the same intensive dietary counselling. The follow-up period of the study was 5 years. Detailed information on the specific recommended diets as well as mean baseline and changes in energy and nutrient intake after the 5-years intervention period for both dietary patterns has been described elsewhere [24].
In this study, although both dietary patterns share common characteristics in key components (e.g., abundant intake of fruit, vegetables, whole grains and legumes), an increase in the consumption of oily fish, nuts, and extra virgin olive oil was found, along with a reduction in the consumption of harmful foods such as red/processed meats and pastries/commercial bakery products in patients adhering to the Mediterranean diet compare to the low-fat diet. Adherence to the Mediterranean diet was determined by the 14-item MEditerranean Diet Adherence Screener (MEDAS) while that of the low-fat diet was evaluated by a 9-item dietary screener. Details of the dietary adherence assessment have been previously published [24].
Anthropometric measurements and laboratory tests
At 8.00 am, following a 12 h fast, the patients were admitted to the laboratory for anthropometric and biochemical tests [weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL-cholesterol, LDL-cholesterol, total cholesterol, triglycerides, high sensitive C-reactive protein (hsCRP), fasting glucose and insulin, homeostasis model assessment of insulin resistance (HOMA-IR) and hemoglobin A1c (HbA1c) as described previously [25]. Serum and urine creatinine were determined by the modified Jaffé colorimetric method [26] and measured by spectrophotometry (BioSystems SA, Barcelona, Spain).
Evaluation of kidney function
Kidney function was evaluated by measuring serum creatinine (sCr)-based eGFR, calculated using the CKD-Epi (CKD Epidemiology Collaboration) equation as previously published [27, 28].
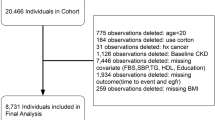
All 1002 patients completed the evaluation of kidney function at baseline (all of them with eGFR ≥ 30 ml/min/1.73 m2, as one of the inclusion criteria of the study [23]). Out of these 1002 patients, 69 did not complete the 5-year follow-up due to withdrawal or death. From these 933 patients, 74 patients had no eGFR data available and 6 patients showed extreme values of total energy intake: < 500 kcal/day or > 3500 kcal/day for women and < 800 kcal/day or > 4000 kcal/day for men, following the criteria proposed by Willet [29]. In this context, the kidney function of 853 patients was evaluated after the 5-year follow-up period. The screening and randomization flow-chart of the CORDIOPREV study and the evaluation of kidney function are shown in Figure S1. Baseline characteristics of those patients who completed the evaluation of kidney function study (during follow-up) compared to patients who did not complete it have been published previously [22].
We also evaluated the ratio of urinary albumin to creatinine (uACR), both at baseline and after the 5-year follow-up period. This ratio was computed by dividing the concentration of urinary albumin concentration by that of creatinine, in mg/g. Using this method, which is based on a spot urine test, produces results which are comparable to those obtained using a 24 h urine collection [30]. Patients who had their kidney function evaluated at 5 years (n = 853, with available eGFR data) also had uACR measurements available both at baseline and after 5 years of dietary intervention.
Criteria for Type 2 diabetes mellitus and obesity status
T2DM was defined according to the American Diabetes Association (ADA) diagnosis criteria [31]. Obesity was defined as a BMI ≥ 30 kg/m2. In order to evaluate the specific contribution of obesity, along with T2DM or not, on eGFR, we classified the population into four different groups: Non-Obesity/Non-T2DM, Obesity/Non-T2DM, Non-Obesity/T2DM and Obesity/T2DM.
Statistical analyses
The data are presented, for the continuous variables, as the mean ± standard error of the mean (SE) and, for the categorical variables, as proportions. The biochemical variables were assessed for normal distribution and variables that presented skewed distribution were normalized by log10 transformation. We compared the categorical variables using Chi-Square tests, and assessed between-group changes with an unpaired t-test or univariate ANOVA for the continuous variables, as required. Significant correlations were studied using bivariate Pearson correlation methods. Test of linear trend across the groups were performed assigning the median value of eGFR and uACR to each group and treating the variable as continuous. To evaluate the variation in data according to diet and time (from baseline to 5 years), we used repeated-measures ANOVA analyses, with a Bonferroni post-hoc test. We compared Δchanges (between 5-year and baseline values) in eGFR between groups with univariate ANOVA adjusted by required cofounders. We conducted a generalized linear model (GLM) using ΔeGFR, as dependent variable, to evaluate potential interaction effects of diet on changes in eGFR according to the presence of diabetes and/or obesity. The effect size was based on GLM coefficients (β) with a 95% confidence interval. The statistical analyses were carried out using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline anthropometric and biochemical characteristics of the study population
The baseline characteristics of patients with CHD whose kidney function was assessed at baseline and who were assigned to randomized dietary groups, have been published previously [22]. We observed that patients who did not undergo the follow-up evaluation of kidney function study were older, with a greater prevalence of current smoking compared to those who completed the assessment [22].
Baseline characteristics of the patients according to presence or absence of obesity are shown in Table S1. Patients with obesity exhibited higher uACR, HbA1c, HOMA-IR and fasting glucose, insulin and hsCRP levels and lower HDL-cholesterol levels compared to patients without obesity (all p < 0.001). No differences in baseline eGFR were observed between patients with and without obesity. Moreover, patients with obesity showed a higher percentage of presence of T2DM and, consequently, higher antidiabetic treatment, compared to patients without T2DM (all p < 0.001).
The baseline characteristics according to the presence or absence of T2DM have been published previously [22]. Briefly, patients with T2DM were older, and contained a higher percentage of patients with obesity and under treatment for diabetes and/or hypertension, compared to patients without T2DM (all p < 0.05). Patients with T2DM also had lower eGFR and higher uACR and HOMA-IR, fasting glucose, insulin, and HbA1c and triglyceride levels in comparison to patients without T2DM (all p < 0.05).
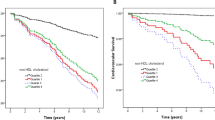
When T2DM and obesity were considered together, these patients (Obesity/T2DM) exhibited the highest HbA1c, HOMA-IR and fasting glucose, insulin, hsCRP and triglyceride levels (all p < 0.001) (Table 1). Regarding the parameters related to kidney function, patients with Obesity/T2DM showed lower eGFR compared to their counterparts without T2DM (p < 0.001) with a significant trend across the groups (p = 0.035) (Fig. 1). Moreover, patients with Obesity/T2DM exhibited the highest uACR (129.00 ± 21.57 mg/g) compared to the other groups (Non-Obesity/Non-T2DM = 15.85 ± 25.55 mg/g; Obesity/Non-T2DM = 21.82 ± 25.83 mg/g, Non-Obesity/T2DM = 58.08 ± 27.04 mg/g; all p < 0.001) with a significant trend across the groups (p < 0.001, Figure S2).
Data are presented as mean ± standard error of the mean. Variables were compared using the analysis of variance (univariate ANOVA) adjusted by hypertension, smoking and drinking habits and use of lipid-lowering drugs. Differences were significant when p < 0.05. Different common letter denote significant differences between groups (p < 0.05). Test of linear trends across the groups were performed assigning the median value of eGFR and uACR to each group and treating the variable as continuous (ptrend). Non-obesity/Non-T2DM (n = 233), Obesity/Non-T2DM (n = 229), Non-obesity/T2DM (n = 208) and Obesity/T2DM (n = 332). CHD coronary heart disease; T2DM, type 2 diabetes mellitus; eGFR, estimated glomerular filtration rate; uACR, urine albumin-creatinine ratio.
Associations between parameters of kidney function and those related to glucose metabolism and obesity
Correlations between kidney function parameters and T2DM and obesity-related parameters at baseline in the total study population are shown in Table S2. We found that baseline eGFR was inversely associated with HbA1c, fasting glucose (all p < 0.001) and insulin levels (p = 0.058) but not with BMI. Moreover, uACR was directly associated with HbA1c, fasting glucose and insulin levels and BMI (all p < 0.001).
Dietary effect on kidney function in presence or absence of T2DM and/or obesity
The effect of dietary intervention (Δ changes produced between post- and pre-intervention) on eGFR according to the presence or absence of T2DM and/or obesity is shown in Fig. 2.
A After both dietary patterns and (B) After each dietary pattern independently. Non-obesity/Non-T2DM (n = 203), Obesity/Non-T2DM (n = 197), Non-Obesity/T2DM (n = 178) and Obesity/T2DM (n = 275). Data are presented as Δ changes in eGFR (mL/min/1.73 m2) produced between post- and preintervention ± standard error of the mean. Variables were compared using the analysis of variance (univariate ANOVA) adjusted by age, sex, baseline hypertension, baseline eGFR categories, anti-diabetic drugs and changes in energy intake. Differences were significant when p < 0.05. * Significant differences between post and pre-intervention.#Significant differences between Mediterranean diet and low-fat diet. Global p-values: p (group): presence in obesity and/or T2DM group effect and p (diet): diet effect. eGFR, estimated glomerular filtration rate; CHD, coronary heart disease; T2DM, type 2 diabetes mellitus.
In all the groups of patients, eGFR declined after dietary intervention period, regardless of the type of diet (Mediterranean diet or low-fat diet), compared to baseline (all p < 0.001) without differences among groups (Fig. 2A). However, when the type of dietary model was considered, consuming the Mediterranean diet led to a lower reduction in eGFR in comparison to the low-fat diet only in patients with Obesity/T2DM (p = 0.032). In fact, after consuming the Mediterranean diet, the decline rate of eGFR was −2.698 ml/min/1.73 m2 lower when compared with the low-fat diet, in this type of patients, during the 5-year follow-up period (p = 0.032) (Fig. 2B).
To evaluate potential interaction effects of diet on changes in eGFR according to the presence of diabetes and/or obesity, we conducted a GLM (Table 2). In patients without T2DM, no significant interaction was found between the diet and the presence of obesity. However, in patients with T2DM, we found a significant interaction between diet and presence of obesity. Patients with obesity consuming the low-fat diet show a β = −3.009, p = 0.033 for changes in eGFR compared to patients with obesity consumed the Mediterranean diet (as reference).
Regarding the analysis of another parameter related to kidney function, uACR, according to the presence/absence of T2DM and obesity, after dietary intervention, we found that the Mediterranean diet reduced uACR (∆uACR −1.62 ± 12.98 mg/g), compared to the low-fat diet (∆uACR 18.51 ± 12.65 mg/g; p = 0.024) in patients with Obesity/T2DM only, but not in the other groups of patients. Moreover, in patients with Obesity/T2DM, the Mediterranean diet was able to maintain fasting glucose levels after 5-years of follow-up, while the low-fat diet increased it. No significant differences were found in the other clinical parameters, according to each dietary pattern, in this group of patients (Table 3). We also found no significant differences in antidiabetic and antihypertensive treatments between baseline and after 5 years of follow-up in each study group of patients and considering each dietary pattern separately (Table S3).
With the aim to determine whether these findings were related to the effect of weight loss, after dietary intervention, we evaluated changes in weight in these patients. However, no significant difference was found in weight loss between the two diets in patients with Obesity/T2DM (Mediterranean diet: ∆weight = −2.83 ± 0.60; low-fat diet: ∆weight = −2.18 ± 0.61 kg, p = 0.450).
Moreover, we wondered if the effect of diet observed in changes in eGFR in patients with Obesity/T2DM could be due to differences in dietary adherence among the groups of patients. For this reason, we evaluated differences in dietary adherence according to the presence of T2DM and/or obesity. Mean baseline values and changes in adherence after 5 years of dietary intervention are shown in Table S4. All the groups of patients showed significant increases in the dietary adherence after 5 years, compared to baseline (all p < 0.05). No differences were found either in baseline values or ∆changes in dietary adherence among the groups of patients.
Discussion
To the best of our knowledge, no previous studies have evaluated the impact of obesity on T2DM and its effect on kidney function in patients with CHD, despite the fact that these patients are at an increased risk for kidney complications. In this randomized and controlled dietary clinical trial, we found that the presence of obesity had an additive effect on T2DM, resulting in a greater impairment of kidney function with lower eGFR compared to their counterparts without T2DM and the highest uACR. Long-term Mediterranean diet consumption, in comparison to a low-fat diet, produced a lower deterioration of kidney function (lower eGFR decline and uACR) only in patients with more metabolic complications (e.g., with concomitant T2DM and obesity). In addition to age, the presence of certain chronic diseases determines a progressive decline in eGFR [32]. In fact, several studies have argued that kidney function is impaired by diabetes, pointing to an average annual decline in eGFR of ≈2 mL/min/1.73 m2 [33]. However, while there is an independent association between obesity and reduced eGFR in individuals with and without CKD [34], other studies suggest that metabolic imbalance contributes more to kidney function impairment than elevated body fat alone [35, 36].
A recent observational study pointed out the presence of metabolic syndrome, rather than obesity measures (BMI or waist circumference), as being associated with an accelerated eGFR decline in the general population [13]. These findings are in line with our results, where patients with T2DM (in the presence of obesity) showed reduced eGFR compared to patients without T2DM patients (with and without obesity). Moreover, eGFR was inversely correlated with HbA1c and fasting glucose levels but not with BMI, suggesting a greater contribution of metabolic imbalance or a loss of metabolic flexibility to kidney dysfunction than the influence of body mass.
The close interconnection between T2DM and obesity can make it difficult to determine the precise impact of each disease on the impairment of kidney function, and as a result, it may be challenging to develop effective therapies and treatments. Over the last few years, lifestyle modifications, particularly dietary habits, have been recognized as the first-line strategies in the management of T2DM and its complications [37, 38]. In fact, in the context of the CORDIOPREV study, we have recently found that the long-term consumption of a healthy dietary pattern (a Mediterranean or a low-fat diet) reduced the risk of developing T2DM in patients with CHD [20, 39]. Moreover, we have also demonstrated that the Mediterranean diet, compared to a low-fat diet, produced a positive effect on delaying the decline of eGFR in patients with CHD. However, when the presence or absence of T2DM was considered, this effect was observed only in those patients with T2DM [22]. In this study, the effect of the Mediterranean diet in slowing down the decline of eGFR was more pronounced when T2DM and obesity coexisted rather than when these pathologies were considered independently, with no differences in weight loss between the diets. Furthermore, no changes in dietary adherence to either diet were detected among the groups, regardless of the presence of diabetes and/or obesity.
While the precise molecular mechanisms remain unclear, we can hypothesize that the impact made by the Mediterranean diet on eGFR-based kidney function in our population could be attributed to its established modulation of cardiometabolic risk factors, such as a better control of plasma lipid levels, reduction of hypertension and attenuation of oxidative stress and inflammation [40,41,42]. Current data suggest that vascular changes associated with endothelial dysfunction play an important role in the progression of renal impairment [43]. In line with this, our recent findings indicate that the Mediterranean diet is capable of restoring endothelial dysfunction through a more evenly balanced endothelial homeostasis [44, 45]. The Mediterranean diet, characterized by an abundance of minimally processed natural foods, together with extra virgin olive oil (EVOO) as the primary source of fat, provides MUFA and other minor components with antioxidant and anti-inflammatory properties. This dietary pattern has been associated with reduced insulin resistance and has improved endothelium-dependent vasodilatation in patients with T2DM [46]. Another possible mechanism by which the Mediterranean diet affects kidney function could be by reducing the circulating levels of advanced glycation end products (AGEs). In fact, our recent studies have demonstrated that consumption of a Mediterranean dietary pattern can lead to reduced circulating AGEs levels in patients with CHD and T2DM, as well as in patients with metabolic syndrome and in elderly adults [25, 47, 48]. These cytotoxic compounds, known for their oxidant and pro-inflammatory properties, play an important role in the pathogenesis of renal disorders, particularly when the patient suffers from T2DM and obesity [49, 50].
Our study has several key strengths. First, it employs a randomized design that encompasses two different dietary patterns with a large sample of patients. Moreover, it is an in-depth dietary intervention study which compares two healthy dietary patterns with an excellent dietary adherence [24, 51]. However, one potential limitation could be the absence of a standard control diet. Nevertheless, as our study was carried out within the framework of the CORDIOPREV trial, which involves patients with diagnosed CHD in the secondary prevention stage, it was not deemed ethically viable to use a control diet during the design phase of the study. After studying a range of guidelines and international consensus documents [52,53,54,55], we opted for testing the long-term effect of two active intervention groups (a low-fat diet vs a Mediterranean diet), both of which had proven benefits in patients with high cardiovascular risk. Nevertheless, the study also has certain limitations. This research is based on a long-term, well-controlled dietary intervention, ensuring the quality of the study, but it may not reflect the level of compliance in a free-living population. Furthermore, the findings are confined to patients with T2DM and CHD, which limits their general applicability to other populations. Additionally, it is important to note that the evaluation of kidney function was not the primary endpoint of the CORDIOPREV study, which means that it was impossible to link causality from our findings. Finally, eGFR was not determined through direct measurement such as inulin or iothalamate, or 24-h urinary creatinine clearance, as these costly and time-consuming procedures are not suited to the routine detection of kidney disease.
In summary, our data provide evidence for the greater contribution of obesity and T2DM combined than of T2DM alone to the impairment of kidney function in patients with CHD compared to patients without T2DM. Our study also supports the notion that the response to two healthy dietary interventions on eGFR differs depending on whether obesity and T2DM coexist or are present separately. We propose that patients with both T2DM and obesity, who also suffer more metabolic complications, may respond better in terms of preserving kidney function to the beneficial effects of the consumption of the Mediterranean diet, compared to a low-fat diet. These findings highlight the potential of dietary strategies as clinical and therapeutic tools, leading to a better understanding of personalized health, particularly, in the context of secondary cardiovascular disease prevention.
Data availability
Collaborations with the CORDIOPREV study are open to Biomedical Institutions, always following an accepted proposal for scientific study. Depending on the nature of the collaboration, electronic data, hard copy data, or biological samples should be provided. All collaborations will be made after a collaboration agreement. Terms of the collaboration agreement will be specific for each collaboration, and the extent of the shared documentation (i.e., anonymized participant data, data dictionary, biological samples, hard copy, or other specified data sets) will also be specifically agreed in the light of each work.
References
Fernandez-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, et al. Importance of Genetic Studies of Cardiometabolic Disease in Diverse Populations. Circ Res. 2020;126:1816–40.
Lagstrom H, Stenholm S, Akbaraly T, Pentti J, Vahtera J, Kivimaki M, et al. Diet quality as a predictor of cardiometabolic disease-free life expectancy: the Whitehall II cohort study. Am J Clin Nutr. 2020;111:787–94.
Sattar N, Gill JMR, Alazawi W. Improving prevention strategies for cardiometabolic disease. Nat Med. 2020;26:320–5.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0158765.
Pecoits-Filho R, Abensur H, Betonico CC, Machado AD, Parente EB, Queiroz M, et al. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr. 2016;8:50.
Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. 2017;55:66–76.
Mohammedi K, Chalmers J, Herrington W, Li Q, Mancia G, Marre M, et al. Associations between body mass index and the risk of renal events in patients with type 2 diabetes. Nutr diabetes. 2018;8:7.
de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2:417–26.
Esmeijer K, Geleijnse JM, Giltay EJ, Stijnen T, Dekker FW, de Fijter JW, et al. Body-fat indicators and kidney function decline in older post-myocardial infarction patients: The Alpha Omega Cohort Study. Eur J Prev Cardiol. 2018;25:90–9.
Akwo EA, Sahinoz M, Alsouqi A, Siew ED, Ikizler TA, Hung AM. Effect Modification of Body Mass Index and Kidney Function on Insulin Sensitivity Among Patients With Moderate CKD and Healthy Controls. Kidney Int Rep. 2021;6:2811–20.
Wallace AS, Chang AR, Shin JI, Reider J, Echouffo-Tcheugui JB, Grams ME, et al. Obesity and Chronic Kidney Disease in US Adults With Type 1 and Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2022;107:1247–56.
Hill CJ, Cardwell CR, Maxwell AP, Young RJ, Matthews B, O’Donoghue DJ, et al. Obesity and kidney disease in type 1 and 2 diabetes: an analysis of the National Diabetes Audit. QJM. 2013;106:933–42.
Stefansson VTN, Schei J, Solbu MD, Jenssen TG, Melsom T, Eriksen BO. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018;93:1183–90.
American Diabetes A. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S89–S97.
Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007.
Esposito K, Maiorino MI, Ciotola M, Di Palo C, Scognamiglio P, Gicchino M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306–14.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl J Med. 2008;359:229–41.
Maroto-Rodriguez J, Ortolá R, Carballo-Casla A, Iriarte-Campo V, Salinero-Fort M, Rodríguez-Artalejo F, et al. Association between a mediterranean lifestyle and Type 2 diabetes incidence: a prospective UK biobank study. Cardiovasc Diabetol. 2023;22:271.
O’Connor LE, Hu EA, Steffen LM, Selvin E, Rebholz CM. Adherence to a Mediterranean-style eating pattern and risk of diabetes in a U.S. prospective cohort study. Nutr diabetes. 2020;10:8.
Roncero-Ramos I, Alcala-Diaz JF, Rangel-Zuniga OA, Gomez-Delgado F, Jimenez-Lucena R, Garcia-Rios A, et al. Prediabetes diagnosis criteria, type 2 diabetes risk and dietary modulation: The CORDIOPREV study. Clin Nutr. 2020;39:492–500.
Roncero-Ramos I, Gutierrez-Mariscal FM, Gomez-Delgado F, Villasanta-Gonzalez A, Torres-Pena JD, Cruz-Ares S, et al. Beta cell functionality and hepatic insulin resistance are major contributors to type 2 diabetes remission and starting pharmacological therapy: from CORDIOPREV randomized controlled trial. Transl Res. 2021;238:12–24.
Podadera-Herreros A, Alcala-Diaz JF, Gutierrez-Mariscal FM, Jimenez-Torres J, Cruz-Ares S, Arenas-de Larriva AP, et al. Long-term consumption of a mediterranean diet or a low-fat diet on kidney function in coronary heart disease patients: The CORDIOPREV randomized controlled trial. Clin Nutr. 2022;41:552–9.
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Perez-Caballero AI, Gomez-Delgado F, et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. Am Heart J. 2016;177:42–50.
Quintana-Navarro GM, Alcala-Diaz JF, Lopez-Moreno J, Perez-Corral I, Leon-Acuna A, Torres-Pena JD, et al. Long-term dietary adherence and changes in dietary intake in coronary patients after intervention with a Mediterranean diet or a low-fat diet: the CORDIOPREV randomized trial. Eur J Nutr. 2020;59:2099–110.
Gutierrez-Mariscal FM, Cardelo MP, de la Cruz S, Alcala-Diaz JF, Roncero-Ramos I, Guler I, et al. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol Nutr Food Res. 2021;65:e1901290.
Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162:548–54.
Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol. 2021;32:2994–3015.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Willet W Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998.
Busby DE, Bakris GL. Comparison of commonly used assays for the detection of microalbuminuria. J Clin Hypertens (Greenwich). 2004;6:8–12.
American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13–S27.
Mallappallil M, Friedman EA, Delano BG, McFarlane SI, Salifu MO. Chronic kidney disease in the elderly: evaluation and management. Clin Pr (Lond). 2014;11:525–35.
Vistisen D, Andersen GS, Hulman A, Persson F, Rossing P, Jorgensen ME. Progressive Decline in Estimated Glomerular Filtration Rate in Patients With Diabetes After Moderate Loss in Kidney Function-Even Without Albuminuria. Diabetes Care. 2019;42:1886–94.
Chang AR, Grams ME, Ballew SH, Bilo H, Correa A, Evans M, et al. Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ. 2019;364:k5301.
Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52:39–48.
Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–6.
Yubero-Serrano EM, Delgado-Lista J, Tierney AC, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, et al. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: the LIPGENE study. Am J Clin Nutr. 2015;102:1509–17.
Sotos-Prieto M, Ortolá R, Ruiz-Canela M, Garcia-Esquinas E, Martínez-Gómez D, Lopez-Garcia E, et al. Association between the Mediterranean lifestyle, metabolic syndrome and mortality: a whole-country cohort in Spain. Cardiovasc Diabetol. 2021;20:5.
Roncero-Ramos I, Jimenez-Lucena R, Alcala-Diaz JF, Vals-Delgado C, Arenas-Larriva AP, Rangel-Zuniga OA, et al. Alpha cell function interacts with diet to modulate prediabetes and Type 2 diabetes. J Nutr Biochem. 2018;62:247–56.
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Perez-Caballero AI, Perez-Jimenez F, Lopez-Miranda J. Mediterranean Diet and Cardiovascular Risk: Beyond Traditional Risk Factors. Crit Rev Food Sci Nutr. 2016;56:788–801.
Gomez-Marin B, Gomez-Delgado F, Lopez-Moreno J, Alcala-Diaz JF, Jimenez-Lucena R, Torres-Pena JD, et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: the Cordioprev randomized trial. Am J Clin Nutr. 2018;108:963–70.
Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, Lopez-Miranda J. Extra virgin olive oil: More than a healthy fat. Eur J Clin Nutr. 2019;72:8–17.
Radenkovic M, Stojanovic M, Prostran M. Endothelial Dysfunction in Renal Failure: Current Update. Curr Med Chem. 2016;23:2047–54.
Torres-Pena JD, Garcia-Rios A, Delgado-Casado N, Gomez-Luna P, Alcala-Diaz JF, Yubero-Serrano EM, et al. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis. 2018;269:50–6.
Yubero-Serrano EM, Fernandez-Gandara C, Garcia-Rios A, Rangel-Zuniga OA, Gutierrez-Mariscal FM, Torres-Pena JD, et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020;17:e1003282.
Ryan M, McInerney D, Owens D, Collins P, Johnson A, Tomkin GH. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM. 2000;93:85–91.
Lopez-Moreno J, Quintana-Navarro GM, Camargo A, Jimenez-Lucena R, Delgado-Lista J, Marin C. et al. Dietary fat quantity and quality modifies advanced glycation end products metabolism in patients with metabolic syndrome. Mol Nutr Food Res. 2017;61:1601029.
Lopez-Moreno J, Quintana-Navarro GM, Delgado-Lista J, Garcia-Rios A, Delgado-Casado N, Camargo A, et al. Mediterranean Diet Reduces Serum Advanced Glycation End Products and Increases Antioxidant Defenses in Elderly Adults: A Randomized Controlled Trial. J Am Geriatr Soc. 2016;64:901–4.
Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63:S39–62.
Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic Toxicity of Advanced Glycation End Products in CKD. J Am Soc Nephrol. 2016;27:354–70.
Delgado-Lista J, Alcala-Diaz JF, Torres-Pena JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399:1876–85.
de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85.
Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl J Med. 2018;378:e34.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e505–e29.
Acknowledgements
We would like to thank the EASP (Escuela Andaluza de Salud Publica), Granada (Spain), for carrying out the randomization process in this study.
Funding
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero (Cordioprev-CEAS, 1/2016 to Jose Lopez-Miranda). This study also received research grants from Ministerio de Ciencia e Innovación (AGL2012-39615, AGL2015-67896-P and PID2019-104362RB-I00 funded by MCIN/AEI/1.0.13039/501100011033 to Jose Lopez-Miranda), from Consejería de Salud-Junta de Andalucía (PC-0283-2017 to Elena M. Yubero-Serrano) and FIS (PI18/01822 and PI21/00383 to Elena M Yubero-Serrano), integrated into the framework of the National Plan for Scientific Research, Technological Development and Innovation 2013-2016, co-financed by the Instituto de Salud Carlos III (ISCIII) of Spain and also by the General Directorate for Assessment and Promotion of Research and the EU’s European Regional Development Fund (FEDER). Elena M Yubero-Serrano was the recipient of the Nicolas Monardes Programme from the “Servicio Andaluz de Salud, Junta de Andalucia”, Spain (C1-0005-2019). The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank the EASP (Escuela Andaluza de Salud Publica), Granada (Spain), for carrying out the randomization process in this study. The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
Author information
Authors and Affiliations
Contributions
APH and APAR was responsible for extracting and analyzing data and writing the original draft. FMGM contributed to data curation and writing the original draft. JFAD and JDTP were responsible for formal analysis and validation. AOR and MPC contributed to analyzing data and validation. FRC and DRC were responsible for determining biochemical data. RML and JMO were responsible for reviewing and supervising the manuscript. PPM contributed to data extraction and provided feedback on the report. JDL contributed to the design of analysis and interpreting results. JLM and EMYS were responsible for the conceptualization of the final manuscript, funding acquisition, and reviewing and final editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The current work was conducted within the framework of the Coronary Diet Intervention with Olive Oil and Cardiovascular Prevention Study (CORDIOPREV; Clinical trials.gov. Identifier: NCT00924937). The trial protocol was approved by the Reina Sofia University Hospital Ethics Committee, following the Helsinki declaration and good clinical practices. All the patients gave their written informed consent to participate in the study. The experimental protocol conformed to international ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Screening and randomization flow-chart of the CORDIOPREV study and the evaluation of kidney function
41387_2024_285_MOESM4_ESM.docx
Correlations between kidney function parameters and T2DM and obesity related parameters at baseline in the total study population.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Podadera-Herreros, A., Arenas-de Larriva, A.P., Gutierrez-Mariscal, F.M. et al. Mediterranean diet as a strategy for preserving kidney function in patients with coronary heart disease with type 2 diabetes and obesity: a secondary analysis of CORDIOPREV randomized controlled trial. Nutr. Diabetes 14, 27 (2024). https://doi.org/10.1038/s41387-024-00285-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-024-00285-3