Abstract
Despite a lack of evidence, patients are often not fed for 48–96 h after upper gastrointestinal bleeding (UGIB); however, many trials have demonstrated the benefits of early nutrition (EN). We conducted a meta-analysis of randomized controlled trials (RTCs) to evaluate the outcomes of EN compared to delayed nutrition (DN) after UGIB. The protocol was registered on PROSPERO (CRD42022372306). PubMed, Embase, CENTRAL, Scopus, and Web of Science were searched on the 27th of April 2024 to identify eligible RCTs. The primary outcomes were early (within 7 days) and late (within 30–42 days) mortality and rebleeding. Pooled risk ratios (RR), mean differences (MD), and corresponding 95% confidence intervals (CI) were calculated using a random-effects model. A total of 10 trials with 1051 patients were included in the analysis. Early mortality was not significantly different between the two groups (RR 1.20, CI 0.85–1.71, I2 = 0%), whereas late mortality was reduced to a clinically relevant extent in the EN group (RR 0.61, CI 0.35–1.06, I2 = 0%). When comparing the two groups, we found no significant difference in terms of early and late rebleeding (RR 1.04, CI 0.66–1.63, I2 = 0% and RR 1.16, CI 0.63–2.13, I2 = 0%, respectively). Our analysis also showed that the length of hospital stay was reduced in the EN group compared to the DN group (MD −1.22 days, CI: −2.43 to −0.01, I2 = 94%). In conclusion, compared with DN, EN (within 24 h) appears to be a safe intervention and could reduce the length of hospital stay without increasing the risk of complications after UGIB.
Similar content being viewed by others
Introduction
Upper gastrointestinal bleeding (UGIB) is a common medical emergency with severe consequences if not managed appropriately. It can be triggered by a variety of underlying causes, such as peptic ulcers and varices. Its incidence is increasing unacceptably, with mortality rates ranging from 2 to 10%1,2,3,4. Regardless of the source, bleeding can lead to malnutrition and other complications that can prolong hospitalization and increase mortality. Therefore, providing adequate nutrition to patients with UGIB is crucial.
Early nutrition (EN) has been suggested to improve outcomes by reducing the risk of infections5 and maintaining gut mucosal integrity. On the other hand, delayed nutrition (DN) has been considered to minimize the risk of rebleeding and other complications arising from introducing food or nutrients too soon after an episode of bleeding6,7. However, it can also lead to malnutrition and delayed recovery.
Based on current research findings, patients at high risk of rebleeding are recommended to abstain from eating and stay hospitalized for at least 48–72 h following endoscopic treatment. During this period, many high-risk lesions are expected to become low-risk, and the majority of rebleeding incidents tend to occur8,9. However, The optimal time to start feeding remains a controversial topic, and the nutrition strategy should be based on endoscopic findings in patients with UGIB6. Patients who have low-risk endoscopic findings, such as clean-based ulcers, can typically resume a regular diet shortly after the endoscopic procedure and may be discharged if there are no other reasons for hospitalization10. However, those with higher-risk endoscopic findings, even after undergoing endoscopic therapy, may require feeding within 72 h, although the exact timing and specific dietary recommendations are uncertain9,11,12. Individual patient factors, such as severity of bleeding, comorbidities, and risk of complications, should also be considered when making treatment decisions. In this context, it is essential to weigh up the potential benefits and risks of early versus delayed nutrition.
Some studies showed that EN could be beneficial in reducing complications in patients with UGIB13,14,15; however, others favored DN7,16. A previous meta-analysis by Zhang et al.17 set out to investigate this clinical question but included only five trials. In contrast, we evaluated a broader spectrum of outcomes and analyzed the early and late rebleeding and mortality separately. In addition, we included five more trials compared to the previously investigated ones. Therefore, we meta-analyzed randomized controlled trials (RCTs) to assess the efficacy and safety of EN compared to DN and grouped them by source of bleeding.
Methods
Our meta-analysis was conducted following the recommendations of the Cochrane Handbook and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Supplementary Material Table S1)18,19. The study protocol was registered on PROSPERO (CRD42022372306) in advance, and we fully adhered to it20.
Eligibility criteria
Only RCTs were included in our analysis. We applied the PICO (patients, intervention, comparison, outcome) framework to establish our eligibility criteria21; patients were upper gastrointestinal bleeders, including variceal and non-variceal bleeding sources, and the intervention was early nutrition (EN) compared to delayed nutrition (DN). Our primary outcomes were early (within 7 days) and late (within 30–42 days) mortality and rebleeding, whereas the length of hospital stay (LOS), transfusion requirement, transfusion rate, and ICU admission were our secondary endpoints. As for post-hoc analysis, we included bacterial infection, new-onset ascites, and hepatic encephalopathy as additional outcomes. Data on rebleeding and mortality outcomes were combined if reported within three, five, or seven days (early: within 7 days), and likewise for late outcomes, which were pooled together if reported within 30 or 42 days. Any definition for early and delayed nutrition was accepted as specified by the included studies.
Information sources
Our systematic search was conducted in five main databases: Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and Web of Science, from inception to 10th November 2022, and we updated the search on the 27th of April 2024. No language or other restrictions were applied. In addition, a backward and forward citation search was performed using a reference-checking tool to identify all potential references that met our eligibility criteria.
Search strategy
Our search key included three domains. The first domain focused on early and delayed nutrition, whereas the second domain included sources of gastrointestinal bleeding (GIB) and their synonyms. The third domain focused on the concept of randomization, as we included only RCTs. For a detailed search key, see Supplementary Material Table S2.
Screening and selection
After a systematic search, the resulting articles were imported into a reference management program (EndNote 20.1, Clarivate Analytics). Duplicate articles with overlapping publication years, authors, and titles were eliminated automatically and manually. Screening and selection were performed by two independent reviewers (M.O. and D.E.F.), first by title and abstract, and then by full text (considering the eligibility criteria). Cohen's kappa coefficient was calculated at both levels of selection to measure the inter-reviewer reliability22. In case of any disagreement, consensus was reached after discussion with the corresponding author (B.E.).
Data extraction
Relevant data from the eligible studies were extracted independently by two authors (M.O. and D.E.F.). Disagreements were resolved by involving the corresponding author (B.E.). All data were manually collected and entered into an Excel spreadsheet (Office 365, Microsoft, Redmond, WA, USA) in preparation for analysis.
Data items
The following data were extracted: first author, year of publications, digital object identifier, study population, geographic location, study design and period, basic demographics (sex and age), source of bleeding, and bleeding severity scores, including Forrest classification for peptic ulcer bleeding (PUB). Child–Pugh score, Model for end-stage liver disease (MELD) score, and size of esophageal varices were also extracted. In addition, we extracted data on the total number of patients in each arm (early and delayed nutrition), the definition of the outcomes of interest, the definition of the interventions in terms of timing and type of diet used.
Risk of bias assessment and quality of evidence
Two independent authors (M.O. and D.E.F.) assessed the methodological quality of each trial using the revised tool for assessing the risk of bias (ROB 2) recommended by the Cochrane Handbook23. A third reviewer resolved potential disagreements (B.T.). Accordingly, the following potential sources of bias were evaluated: bias due to the randomization process, bias due to deviations from the planned interventions, bias due to missing outcome data, and bias in the measurement of the outcome or the selection of the reported results. We used the robvis (Risk-Of-Bias VISualization) tool to create risk-of-bias plots24.
To assess the quality of evidence for our results, we followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach25, and used the GRADEpro Guideline Development Tool (software) to produce the summary tables of findings. The determinants were study design, risk of bias, inconsistency, indirectness, and imprecision.
Statistical synthesis
The minimum number of studies needed to perform the meta-analysis was three. As we assumed considerable between-study heterogeneity in all cases, a random-effects model was used to pool effect sizes. Risk ratios (RR) with 95% confidence intervals (CI) were used to measure effect size for dichotomous outcomes. For continuous outcomes, the mean difference (MD) was used to measure effect size. To calculate study RR and pooled RR, the total number of patients and the total number of patients with the event of interest in each group were extracted separately from the studies. We reported the results as the risk of an event of interest in the EN group versus the risk of an event of interest in the DN group. To calculate the pooled MD, the mean and standard deviation (SD) were extracted from each study. The studies by Jatin et al.15 and Gong et al.16 reported the LOS using median and SD; therefore, we performed the analysis without them, and included them in a separate analysis assuming a symmetrical distribution where the mean was equal to the median (as the other study reported using mean and SD, we assumed that this was acceptable). The MD was expressed as the mean of the EN group minus the mean of the DN group.
The pooled RR was calculated using the Mantel–Haenszel method26,27. The exact Mantel–Haenszel method (without continuity correction) was used to handle zero cell counts as recommended28. The inverse variance weighting method was used to calculate the pooled MD. We used a Hartung–Knapp adjustment for CIs29,30. To estimate the heterogeneity variance measure (\({\tau }^{2}\)) for RR calculation, the Paule–Mandel method31 was used. For MD calculation, the restricted maximum-likelihood estimator was used with the Q profile method for CI. Prediction interval calculations were based on t-distribution.
Results were considered statistically significant if the CI did not contain the null value. We summarized the findings for the meta-analysis in forest plots. In forest plots, for a cell count of zero, the RR of each study with 95% CI was calculated by adding 0.5 as continuity correction (it was used only for the forest plots). Where applicable (the study number was large enough and not too heterogeneous), we reported the prediction intervals for the results. In addition to \({\tau }^{2}\), heterogeneity was assessed using Higgins and Thompson I2 statistics32. The statistical analysis of the data was conducted using the R software (R Core Team, 2019, Vienna, Austria).
For subgroup analyses, we used a fixed-effects “plural” model (mixed-effects model). We assumed different \({\tau }^{2}\) values in the subgroups. To assess the difference between the subgroups, the Cochran’s Q test was used. The null hypothesis was rejected at a 5% significance level. The subgroup analysis by source of bleeding was planned before the data collection. Publication bias in small studies was assessed by visual inspection of Funnel-plots and calculation of p-values for Harbord (for RR)33 and Egger (for MD) tests34. We planned to assume potential bias in small studies if the p-value was less than 10%. (Although we kept in mind that the test had limited diagnostic assessment below 10 studies). Potential outlier publications were explored using different influence measures and plots following the recommendation of Harrer et al.35.
All statistical analyses were performed with R (v4.1.2) using the meta36 (v6.1.0) package for basic meta-analysis calculations and plots, and the dmetar37 (v0.0.9000) package for additional influential analysis calculations and plots.
Ethical approval
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in our study design, conduct, or interpretation. The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
Results
Search and selection
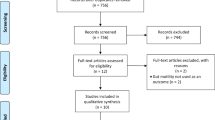
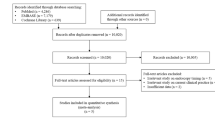
Altogether, 1721 records were identified in the five databases, 904 in Embase, 211 in MEDLINE (via PubMed), 340 in CENTRAL, 101 in Scopus, and 165 in Web on Science. After duplicate removal, 1276 records remained for title and abstract selection. A total of 15 studies were assessed for full-text eligibility, of which six were excluded38,39,40,41,42,43. Five of the six excluded studies were duplicates but with different titles38,40,41,42,43, and one had an ineligible study design39. In addition, we identified 391 records through citation chasing, but only one study was sought for retrieval and was eligible for data extraction44. For more details on our search and selection process, see the PRISMA flowchart (Fig. 1).
Basic characteristics of studies included
Our study consisted of 10 RCTs with a total of 1051 patients5,7,13,14,15,16,44,45,46,47. One of these studies was published as a conference abstract47. Five of the 10 studies focused on patients with non-variceal upper gastrointestinal bleeding (NVUGIB), mainly PUB13,16,45,46,47, whereas the other five focused on patients with VUGIB5,7,14,15,44. The studies were conducted in various geographical locations, five in Asia, three in Europe, one in Africa, and one in North America. More information on the trials, including their basic characteristics, can be found in (Table 1).
Early rebleeding (within 7 days)
We analyzed eight trials5,7,13,14,15,16,44,45 that reported rebleeding within seven days, involving 923 patients (465 in the EN group and 458 in the DN group). In the VUGIB subgroup, our analysis showed no significant difference between the two groups (RR 1.48, 95% CI 0.38–5.71), similarly in the PUB subgroup (RR 0.95, 95% CI 0.54–1.68). Overall, EN did not significantly or relevantly increase the risk of early rebleeding compared to DN (RR 1.04, 95% CI 0.66–1.63, p = 0.845, I2 = 0%, 95% CI 0–68%) (Fig. 2).
Late rebleeding (within 30–42 days)
This analysis included eight studies5,7,14,15,16,44,46,47 that reported rebleeding within 30, 35, or 42 days, involving 693 patients (347 in the EN group and 346 in the DN group). The results were not statistically significant for either subgroup, including PUB (RR 1.14, 95% CI 0.16–7.98) and VUGIB (RR 1.13, 95% CI 0.40–3.17). Overall, EN did not increase the risk of late rebleeding compared to DN (RR 1.16, 95% CI 0.63–2.13, p = 0.583, I2 = 0%, 95% CI 0–68%) (Fig. 3).
Early mortality (within 7 days)
Only five studies7,13,15,44,45 reporting mortality within seven days were included in this analysis, with a total of 543 patients (234 in the EN group and 229 in the DN group). There were no statistically significant differences between the studies in the PUB and VUGIB subgroups (RR 0.98, 95% CI 0.85–1.14, and RR 1.36, 95% CI 0.63–2.91, respectively). The overall effect was not statistically significant between the two groups (RR 1.20, 95% CI 0.85–1.71, p = 0.214, I2 = 0%, 95% CI 0–79%) (Fig. 4).
Late mortality (within 30–42 days)
The analysis included seven studies5,7,14,15,16,44,47 that reported mortality within 30, 35, or 42 days. Altogether, 667 patients were involved (335 in the EN group and 332 in the DN group). There was no statistical difference in either subgroup; in the PUB subgroup (RR 0.51, 95% CI 0.03–7.83) and the VUGIB subgroup (RR 0.73, 95% CI 0.26–2.02). Overall, there was no statistically significant difference between the two groups; however, the results were clinically relevant with a tendency towards the EN group (RR 0.61, 95% CI 0.35–1.06, p = 0.072, I2 = 0%, 95% CI 0–71%) (Fig. 5).
Length of hospital stay (days)
This analysis included six studies5,7,13,14,45,46 involving 570 patients (289 in the EN group and 281 in the DN group). In the PUB subgroup, there was no statistically significant difference between the two groups (MD: −1.34 days, 95% CI − 5.01 to 2.33), whereas in the VUGIB subgroup, EN significantly decreased LOS (MD −1.54 days, 95% CI −2.67 to −0.41). Overall, EN reduced the LOS compared to DN (MD −1.22 days, 95% CI −2.43 to −0.01, p = 0.049, I2 = 94%, 95% CI 90–97%) (Fig. 6).
Jatin et al.15 and Gong et al.16 reported their results in medians instead of means. We decided to exclude those studies from our final analysis due to potentially biased results. However, even if we estimated the means from the provided medians, the findings indicate a significant reduction in LOS (MD −1.12 days, 95% CI −2.03 to −0.22, p = 0.022, I2 = 93%, 95% CI 88–96%) (Supplementary Material Fig. S1).
Blood transfusion requirement (units)
Seven studies5,7,13,14,16,45,46 reported transfusion requirement as an outcome; however, we could analyze only four studies (292 in the EN group and 286 in the DN group)5,16,45,46 due to heterogeneous definitions of this outcome among the included studies (summarized in the Supplementary Material Table S3). Overall, there was no statistically significant difference between the two groups (MD 0.00, 95% CI −0.04 to 0.05, p = 0.980, I2 = 0%, 95% CI 0–85%). (Supplementary Material Fig. S2).
Bacterial infection
Only three studies5,14,15 reported new-onset bacterial infections, including 251 patients (128 in the EN group and 123 in the DN group). Overall, there was no statistically significant difference between the two groups (RR 0.48, 95% CI 0.08–3.05, p = 0.229, I2 = 0%, 95% CI 0–90%) (Supplementary Material Fig. S3).
Ascites and hepatic encephalopathy
Three studies reported on new-onset ascites5,14,15. Overall, there was a tendency that ascites was more common in the DN group, however, it was not statistically significant (RR 0.64, 95% CI 0.34–1.20, p = 0.094, I2 = 0%, 95% CI 0–90%) (Supplementary Material Fig. S4). In addition, two studies5,15 reported on new-onset hepatic encephalopathy. (RR 1.03, 95% CI 0.50–2.11 and RR 0.75, 95% CI 0.18–3.14, respectively). We were not able to draw a statistical inference based on only two studies; therefore, we presented this outcome in the Supplementary Material Fig. S5 without an overall effect.
ICU admission and transfusion rate
We were not able to come to any statistical conclusions regarding these outcomes. Only one study45 reported the ICU admission days; there was no difference between the two groups (EN: 1.1 ± 0.2, DN: 1.1 ± 0.1), and one study15 reported the need for ICU admission (EN: 1/40, DN: 2/40). No studies reported data on the transfusion rate.
Risk of bias assessment and quality of evidence
In the articles, the randomization process and the selection of the reported result domains raised "some concerns". Deviations from the planned intervention and missing outcome data had the lowest risk of bias. Bias from outcome measurement was high in LOS and new-onset hepatic encephalopathy outcomes. Results of risk of bias assessment for all the included studies by outcome are presented in the Supplementary Material Figs. S6–S12.
The quality of evidence was low or very low for all our outcomes. A summary table and explanation of the results can be found in the Supplementary Material (Supplementary Tables S4–S5).
Heterogeneity and publication bias
All analyses of the outcomes included showed negligible statistical heterogeneity levels (I2), with heterogeneity of 0% or less than 10%, except for the LOS outcome, which was 94% (CI 90–97%). This discrepancy may be attributed to variations in bleeding severity among patients, impacting hospitalization needs. We realized that when omitting Laine et al. study45 with leave-one-out sensitivity analysis, which included less severe patients compared to other studies, the effect size became more significant (MD −1.65 days, 95% CI −1.99 to − 1.31), and heterogeneity decreased to 5% (Supplementary Fig. S13–14). As for publication bias, the test's diagnostic accuracy is limited for studies below 10, and as none of our analyses met this threshold, we opted to exclude this analysis.
Discussion
Our study found no significant difference in early and late rebleeding and mortality between EN and DN after UGIB hemostasis; however, these findings are clinically relevant. The results showed that EN could significantly decrease LOS compared to DN. In addition, there was no difference between the two groups in terms of blood transfusion requirement and bacterial infection (Supplementary Material Fig. S15).
Despite the advances in intensive care technologies and improvements in the endoscopic treatment of GIB, it remains a life-threatening emergency with considerable mortality1. Our study revealed a late mortality ratio of 6.89% (46 out of 667 cases), indicating an important concern; we reached a similar conclusion in terms of late rebleeding, with a ratio of 6.78% (47 out of 693 cases).
The precise definitions of both interventions (early and delayed nutrition) played an essential role in determining the timing, dietary type, procedure, and requirements, particularly because there were variations in how these interventions were defined across the included studies. To address this, we have compiled a comprehensive overview of the definitions for both interventions, which can be found in the Supplementary Material Table S6.
According to the current literature, patients at a high risk of rebleeding should be advised to fast and remain hospitalized for a minimum of 48–72 h after endoscopic treatment. Within this timeframe, most high-risk lesions will transition into low-risk lesions, and most rebleeding events will occur8. Therefore, prolonged fasting can be justified. Furthermore, a retrospective study48 showed that delaying refeeding in patients with low-risk lesions who should have been fed promptly is not advisable, and early refeeding is recommended for NVUGIB patients. According to a recent review11, the timing of initiating feeding after the diagnosis of UGIB should be determined by considering patient-specific risk factors associated with the underlying disease. For low-risk patients, it is advisable to resume feeding without delay following endoscopy, as these bleeds are often self-limited and rarely require intervention. However, for higher-risk lesions (Forrest Ia–IIb), the available data on the safety of early refeeding are inconclusive11. This was also a challenge in our analysis, as different studies included patients with varying severities. For example, Gong et al.16 included FIa–FIb bleeders, whereas Khoshbaten et al.13 did not.
Enteral nutrition has the potential to provide several benefits. These include the delivery of local nutrition directly to the gastric tissue, stimulating mucus glands and epithelial cells to support the maintenance of the protective mucus barrier, and promoting increased blood flow to the splanchnic region, which can aid ulcer healing8. In addition, a prospective study49 aimed to compare the early and late postoperative oral feeding of gastric cancer patients undergoing surgery for the recovery of gastrointestinal function. It was found that initiating early oral feeding in patients with gastric cancer facilitates the recovery of postoperative gastrointestinal function without increasing the rate of associated complications or adverse events. Another meta-analysis50 concluded that, in comparison to traditional oral feeding, early refeeding after upper gastrointestinal surgery could shorten the LOS and time of first exhaust without increasing postoperative complications, while also reducing the risk of pneumonia.
Several reviews51,52,53 suggest that enteral nutrition may protect against stress ulceration. Numerous studies in basic science indicate that enteral nutrition can enhance mucosal blood flow and reverse the production of inflammatory mediators53. In addition, the results of a meta-analysis54 indicated that stress ulcer prophylaxis with a histamine-2 receptor blocker may not be necessary for patients receiving enteral nutrition. They found that prophylactic use of a histamine-2 receptor blocker for stress ulcer prevention resulted in a decreased risk of GIB with an odds ratio (OR) of 0.47 (95% CI 0.29–0.76; p < 0.002) However, this treatment effect was observed only in patients who did not receive enteral nutrition. Among patients who were fed enterally, stress ulcer prophylaxis did not have a significant impact on the risk of GIB (OR 1.26; 95% CI 0.43–3.7).
In contrast to the meta-analysis by Zhang et al.17, our study extended their research by including five more clinical trials and examining a broader range of outcomes. Furthermore, we conducted a subgroup analysis based on the source of bleeding, which allowed for more accurate and specific data in our investigation. Their findings also suggested that EN administered within 24 h did not show a higher risk of rebleeding and mortality compared to DN for patients with UGIB. However, EN was associated with a reduction in the LOS.
Risk-stratification systems have been developed to differentiate between patients with a high or low risk of mortality or rebleeding in cases of GIB. However, many of these scores rely on endoscopic findings, which makes them less suitable for early patient evaluation. Fortunately, several risk scores, such as the AIMS65 and Glasgow–Blatchford scores, can be used prior to endoscopy. Therefore, it is crucial to introduce these risk-stratification systems into clinical practice and apply them to determine the optimal timing for initiating enteral nutrition55,56,57.
Regarding the strengths of our analysis, we strictly adhered to our protocol, which was registered beforehand. Our study is the most recent comprehensive analysis of refeeding strategies after UGIB using a rigorous methodology and including only RCTs. In addition, we performed a subgroup analysis based on bleeding source, providing more detailed data.
As for the limitations of this work, only a few studies with a low number of cases could be included. In addition, the EN and DN definitions varied among the studies, and different nutrition modalities and regimens were used. Generalizing the findings might be challenging due to variations in the severity of bleeding among the included patients, which could impact the appropriate timing for refeeding. Other limitations include a high risk of bias in some of the domains and the low quality of evidence.
Our results suggest that EN is a safe intervention; however, further high-quality prospective data collection and reporting are needed to assess this clinical question more accurately, including clinical trials reporting the investigated outcomes based on the severity assessment with longer follow-up periods, others on the diet types and their effects on new-onset ascites, and hepatic encephalopathy might give additional insight into this field. In addition, the emphasis on adherence to risk stratification scores prior to endoscopy ensures appropriate management of those patients58,59.
Conclusion
In comparison to delayed nutrition, early nutrition (within 24 h) appears to be a safe intervention and could reduce the length of hospital stay without increasing the risk of complications in terms of rebleeding or mortality after hemostasis of upper gastrointestinal bleeding.
Data availability
All the data analyzed in this study are available in the full text of the included studies and supplementary material.
References
Oakland, K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol. 42–43, 101610. https://doi.org/10.1016/j.bpg.2019.04.003 (2019).
Zheng, N. S., Tsay, C., Laine, L. & Shung, D. L. Trends in characteristics, management, and outcomes of patients presenting with gastrointestinal bleeding to emergency departments in the United States from 2006 to 2019. Aliment Pharmacol. Ther. 56, 1543–1555. https://doi.org/10.1111/apt.17238 (2022).
Marmo, R. et al. Predicting mortality in patients with in-hospital nonvariceal upper GI bleeding: A prospective, multicenter database study. Gastrointest. Endosc. 79, 741-749.e741. https://doi.org/10.1016/j.gie.2013.10.009 (2014).
Marmo, R. et al. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: A multicenter study. Am. J. Gastroenterol. 103, 1639–1647. https://doi.org/10.1111/j.1572-0241.2008.01865.x (2008) (quiz 1648).
Sidhu, S. S. et al. Early feeding after esophageal variceal band ligation in cirrhotics is safe: randomized controlled trial. Dig. Endosc. https://doi.org/10.1111/den.13423 (2019).
Hebuterne, X. & Vanbiervliet, G. Feeding the patients with upper gastrointestinal bleeding. Curr. Opin. Clin. Nutr. Metab. Care 14, 197–201. https://doi.org/10.1097/MCO.0b013e3283436dc5 (2011).
de Lédinghen, V. et al. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig. Dis. Sci. 42, 536–541. https://doi.org/10.1023/a:1018838808396 (1997).
Rassameehiran, S., Nugent, K. & Rakvit, A. When should a patient with a nonvariceal upper gastrointestinal bleed be fed?. South. Med. J. 108, 419–424. https://doi.org/10.14423/SMJ.0000000000000314 (2015).
Shung, D. L. & Laine, L. Review article: Upper gastrointestinal bleeding—Review of current evidence and implications for management. Aliment Pharmacol. Ther. 59, 1062–1081. https://doi.org/10.1111/apt.17949 (2024).
Barkun, A. N. et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the international consensus group. Ann. Intern. Med. 171, 805–822. https://doi.org/10.7326/M19-1795 (2019).
Busch, R. A., Collier, B. R. & Kaspar, M. B. When can we feed after a gastrointestinal bleed?. Curr. Gastroenterol. Rep. 24, 18–25. https://doi.org/10.1007/s11894-022-00839-4 (2022).
Kaplan, D. E. et al. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology 79, 1180–1211. https://doi.org/10.1097/HEP.0000000000000647 (2024).
Khoshbaten, M., Ghaffarifar, S., Jabbar Imani, A. & Shahnazi, T. Effects of early oral feeding on relapse and symptoms of upper gastrointestinal bleeding in peptic ulcer disease. Dig. Endosc. 25, 125–129. https://doi.org/10.1111/j.1443-1661.2012.01347.x (2013).
Lo, G. H., Lin, C. W. & Hsu, Y. C. A controlled trial of early versus delayed feeding following ligation in the control of acute esophageal variceal bleeding. J. Chin. Med. Assoc. JCMA 78, 642–647. https://doi.org/10.1016/j.jcma.2015.07.004 (2015).
Jatin, Y. et al. An open-label randomized controlled trial of early initiation of nasogastric feeding after endotherapy in variceal bleeding: A proof-of-concept study. J. Clin. Exp. Hepatol. https://doi.org/10.1016/j.jceh.2023.07.413 (2023).
Gong, E. J. et al. Optimal timing of feeding after endoscopic hemostasis in patients with peptic ulcer bleeding: A randomized, noninferiority trial (CRIS KCT0001019). Am. J. Gastroenterol. 115, 548–554. https://doi.org/10.14309/ajg.0000000000000584 (2020).
Zhang, H. et al. Early enteral nutrition versus delayed enteral nutrition in patients with gastrointestinal bleeding A PRISMA-compliant meta-analysis. Medicine (US). https://doi.org/10.1097/MD.0000000000014864 (2019).
Chandler, J. & Hopewell, S. Cochrane methods—twenty years experience in developing systematic review methods. Syst. Rev. 2, 76. https://doi.org/10.1186/2046-4053-2-76 (2013).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Booth, A. et al. PROSPERO at one year: An evaluation of its utility. Syst. Rev. 2, 4. https://doi.org/10.1186/2046-4053-2-4 (2013).
Munn, Z., Stern, C., Aromataris, E., Lockwood, C. & Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 18, 5. https://doi.org/10.1186/s12874-017-0468-4 (2018).
McHugh, M. L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 22, 276–282 (2012).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. https://doi.org/10.1136/bmj.l4898 (2019).
McGuinness, L. A. & Higgins, J. P. T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 12, 55–61. https://doi.org/10.1002/jrsm.1411 (2021).
Guyatt, G. et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, 383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026 (2011).
Mantel, N. & Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748 (1959).
Robins, J., Greenland, S. & Breslow, N. E. A general estimator for the variance of the Mantel–Haenszel odds ratio. Am. J. Epidemiol. 124, 719–723. https://doi.org/10.1093/oxfordjournals.aje.a114447 (1986).
Sweeting, M. J., Sutton, A. J. & Lambert, P. C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 23, 1351–1375. https://doi.org/10.1002/sim.1761 (2004).
IntHout, J., Ioannidis, J. P. & Borm, G. F. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian–Laird method. BMC Med. Res. Methodol. 14, 25. https://doi.org/10.1186/1471-2288-14-25 (2014).
Knapp, G. & Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 22, 2693–2710. https://doi.org/10.1002/sim.1482 (2003).
Paule, R. C. & Mandel, J. Consensus values and weighting factors. J. Res. Natl. Bur. Stand. 1977(87), 377–385. https://doi.org/10.6028/jres.087.022 (1982).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. https://doi.org/10.1002/sim.1186 (2002).
Harbord, R. M., Harris, R. J. & Sterne, J. A. C. Updated tests for small-study effects in meta-analyses. Stata J. 9, 197–210. https://doi.org/10.1177/1536867x0900900202 (2009).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. https://doi.org/10.1136/bmj.315.7109.629 (1997).
Harrer, M. Doing Meta-analysis with R: A Hands-on Guide, 1st edn (CRC Press/Taylor & Francis Group, 2022).
Schwarzer, G. General Package for Meta-Analysis. (2023).
Ebert, M. H. A. P. C. A. T. F. A. D. D. Companion R Package For The Guide 'Doing Meta-Analysis in R. (2019).
Goyal, O. et al. Comparison of effect of early versus delayed feeding on rebleeding following endoscopic variceal band ligation. Hepatol. Int. 13, S219–S220. https://doi.org/10.1007/s12072-019-09936-5 (2019).
Huang, S. Y. & Wang, H. Y. Post esophageal variceal ligation feeding time survey. Hepatol. Int. 6, 298–299. https://doi.org/10.1007/s12072-011-9333-4 (2012).
De Ledinghen, V. et al. The effects of enteral nutrition in immediate decrement of gastrointestinal hemorrhage in cirrhotic patients: A randomized, controlled study. Gastroenterol. Clin. Biol. 20, A120 (1996).
De, L. V. et al. When is time feeding in patients with bleeding peptic ulcer? A randomized controlled study: Quand FAUT-IL Reprendre L’alimentation Orale Apres Hemorragie Ulcereuse Gastro-Duodenale? Etude Controlee Randomisee. Gastroenterol. Clin. Biol. 22, 282–285 (1998).
DeLedinghen, V. et al. Effects of early enteral nutrition in cirrhotics patients after bleeding from esophageal varices: A randomized controlled study. Gastroenterology 110, A13–A13 (1996).
de Ledinghen, V. et al. Early feeding in patients with bleeding peptic ulcer: A randomized controlled study. Gastroenterology 110, A12–A12 (1996).
Goda, T., Mokhtar, A.-R., Anwar, R., Hazem, H. & Eleraki, A. Effect of early versus delayed feeding following emergency endoscopic therapy for acute esophageal variceal bleeding on short-term outcomes. Egypt. J. Intern. Med. 30, 110–114. https://doi.org/10.4103/ejim.ejim_22_18 (2018).
Laine, L. et al. Prospective evaluation of immediate versus delayed refeeding and prognostic value of endoscopy in patients with upper gastrointestinal hemorrhage. Gastroenterology 102, 314–316. https://doi.org/10.1016/0016-5085(92)91816-m (1992).
de Lédinghen, V. et al. When should patients with bleeding peptic ulcer resume oral intake? A randomized controlled study. Gastroenterol. Clin. Biol. 22, 282–285 (1998).
Hepworth, C. C. et al. Randomized controlled trial of early feeding in patients with bleeding peptic-ulcer and a visible vessel. Gastroenterology 108, A113–A113 (1995).
Fonseca, J., Meira, T., Nunes, A. & Santos, C. A. Bleeding and starving: Fasting and delayed refeeding after upper gastrointestinal bleeding. Arq. Gastroenterol. 51, 128–132. https://doi.org/10.1590/s0004-28032014000200011 (2014).
Gao, L., Zhao, Z., Zhang, L. & Shao, G. Effect of early oral feeding on gastrointestinal function recovery in postoperative gastric cancer patients: A prospective study. J. B.U.ON. 24, 181–187 (2019).
Deng, H., Li, B. & Qin, X. Early versus delay oral feeding for patients after upper gastrointestinal surgery: A systematic review and meta-analysis of randomized controlled trials. Cancer Cell Int. https://doi.org/10.1186/s12935-022-02586-y (2022).
MacLaren, R., Jarvis, C. L. & Fish, D. N. Use of enteral nutrition for stress ulcer prophylaxis. Ann. Pharmacother. 35, 1614–1623. https://doi.org/10.1345/aph.1A083 (2001).
Pilkington, K. B., Wagstaff, M. J. & Greenwood, J. E. Prevention of gastrointestinal bleeding due to stress ulceration: A review of current literature. Anaesth. Intensive Care 40, 253–259. https://doi.org/10.1177/0310057X1204000207 (2012).
Hurt, R. T. et al. Stress prophylaxis in intensive care unit patients and the role of enteral nutrition. J. Parenter. Enter. Nutr. 36, 721–731. https://doi.org/10.1177/0148607112436978 (2012).
Marik, P. E., Vasu, T., Hirani, A. & Pachinburavan, M. Stress ulcer prophylaxis in the new millennium: A systematic review and meta-analysis. Crit. Care Med. 38, 2222–2228. https://doi.org/10.1097/CCM.0b013e3181f17adf (2010).
Gralnek, I. M. et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 54, 1094–1120. https://doi.org/10.1055/a-1939-4887 (2022).
Gralnek, I. M. et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 53, 300–332. https://doi.org/10.1055/a-1369-5274 (2021).
Laine, L., Barkun, A. N., Saltzman, J. R., Martel, M. & Leontiadis, G. I. ACG clinical guideline: Upper gastrointestinal and ulcer bleeding. Am. J. Gastroenterol. 116, 899–917. https://doi.org/10.14309/ajg.0000000000001245 (2021).
Hegyi, P., Eross, B., Izbeki, F., Parniczky, A. & Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 27, 1317–1319. https://doi.org/10.1038/s41591-021-01458-8 (2021).
Hegyi, P. et al. Academia Europaea position paper on translational medicine: The cycle model for translating scientific results into community benefits. J. Clin. Med. https://doi.org/10.3390/jcm9051532 (2020).
Funding
Open access funding provided by Semmelweis University. Funding was provided by the ÚNKP-22-3 New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development, and Innovation Fund (to B.T.—ÚNKP-22-3-I-PTE-1693). The study funder had no role in the study design, data collection, data analysis, data interpretation, or the report writing.
Author information
Authors and Affiliations
Contributions
M.O.: conceptualization, investigation, project administration, methodology, visualization, validation, writing—original draft; B.T.: conceptualization, methodology, writing—review and editing; D.E.F.: conceptualization, investigation, writing—review and editing; D.S.V.: conceptualization, formal analysis, software, writing—review and editing; P.H.: conceptualization, validation, writing—review and editing; B.E.: conceptualization, methodology, supervision, validation, writing—original draft. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All authors listed on the title page have read and approved the submission of this manuscript to the Scientific Reports. Authors confirm that the manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obeidat, M., Teutsch, B., Floria, DE. et al. Early nutrition is safe and does not increase complications after upper gastrointestinal bleeding—a systematic review and meta-analysis of randomized controlled trials. Sci Rep 14, 10725 (2024). https://doi.org/10.1038/s41598-024-61543-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61543-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.