Abstract
Elevated lipoprotein (a) (Lp(a)) is associated with premature atherosclerotic cardiovascular disease. However, fewer than 0.5% of individuals undergo Lp(a) testing, limiting the evaluation and use of novel targeted therapeutics currently under development. Here we describe the development of a machine learning model for targeted screening for elevated Lp(a) (≥150 nmol l−1) in the UK Biobank (N = 456,815), the largest cohort with protocolized Lp(a) testing. We externally validated the model in 3 large cohort studies, ARIC (N = 14,484), CARDIA (N = 4,124) and MESA (N = 4,672). The model, Algorithmic Risk Inspection for Screening Elevated Lp(a) (ARISE), reduced the number needed to test to find one individual with elevated Lp(a) by up to 67.3%, based on the probability threshold, with consistent performance across external validation cohorts. ARISE could be used to optimize screening for elevated Lp(a) using commonly available clinical features, with the potential for its deployment in electronic health records to enhance the yield of Lp(a) testing in real-world settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The analyzed de-identified data are available for the UKB cohort from the Access Management System of the UKB and for the ARIC, CARDIA and MESA cohorts from the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the National Heart, Lung, and Blood Institute. The source data for Fig. 6 are available from the corresponding author upon request.
Code availability
Custom code used in this study is available at https://github.com/CarDS-Yale/ARISE.
References
Gilliland, T. C. et al. Lipoprotein(a), oxidized phospholipids, and coronary artery disease severity and outcomes. J. Am. Coll. Cardiol. 81, 1780–1792 (2023).
Nurmohamed, N. S. et al. Finding very high lipoprotein(a): the need for routine assessment. Eur. J. Prev. Cardiol. 29, 769–776 (2022).
The Emerging Risk Factors Collaboration Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 302, 412–423 (2009).
Paré, G. et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 139, 1472–1482 (2019).
Langsted, A., Kamstrup, P. R. & Nordestgaard, B. G. High lipoprotein(a) and high risk of mortality. Eur. Heart J. 40, 2760–2770 (2019).
Nave, A. H. et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis 242, 496–503 (2015).
Patel, A. P. et al. Lp(a) (lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large national biobank. Arterioscler. Thromb. Vasc. Biol. 41, 465–474 (2021).
Trinder, Mark et al. Repeat measures of lipoprotein(a) molar concentration and cardiovascular risk. J. Am. Coll. Cardiol. 79, 617–628 (2022).
Rifai, N., Heiss, G. & Doetsch, K. Lipoprotein(a) at birth, in blacks and whites. Atherosclerosis 92, 123–129 (1992).
Utermann, G. The mysteries of lipoprotein(a). Science 246, 904–910 (1989).
Wilson, D. P. et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 13, 374–392 (2019).
Willeit, P. et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J. Am. Coll. Cardiol. 64, 851–860 (2014).
Kelsey, M. D. et al. Contemporary patterns of lipoprotein(a) testing and associated clinical care and outcomes. Am. J. Prev. Cardiol. 14, 100478 (2023).
Stürzebecher, P. E., Schorr, J. J., Klebs, S. H. G. & Laufs, U. Trends and consequences of lipoprotein(a) testing: cross-sectional and longitudinal health insurance claims database analyses. Atherosclerosis 367, 24–33 (2023).
Bhatia, H. S., Hurst, S., Desai, P., Zhu, W. & Yeang, C. Lipoprotein(a) testing trends in a large academic health system in the United States. J. Am. Heart Assoc. 12, e031255 (2023).
O’Donoghue, M. L. et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2211023 (2022).
Tsimikas, S. et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 382, 244–255 (2020).
Amgen Olpasiran trials of cardiovascular events and lipoprotein(a) reduction (OCEAN(a))—outcomes trial. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT05581303 (2022).
Novartis Pharmaceuticals Assessing the impact of lipoprotein (a) lowering with pelacarsen (TQJ230) on major cardiovascular events in patients with CVD (Lp(a)HORIZON). Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04023552 (2019).
Tsimikas, S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 69, 692–711 (2017).
Varvel, S., McConnell, J. P. & Tsimikas, S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler. Thromb. Vasc. Biol. 36, 2239–2245 (2016).
Reyes-Soffer, G. et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 42, e48–e60 (2022).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139, e1082–e1143 (2019).
Kelsey, M. et al. Lipoprotein(a) testing patterns in a large health system. Am. J. Cardiol. 153, 43–50 (2021).
Kronenberg, F. et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur. Heart J. 43, 3925–3946 (2022).
Khera, R. et al. Multinational patterns of second line antihyperglycaemic drug initiation across cardiovascular risk groups: federated pharmacoepidemiological evaluation in LEGEND-T2DM. BMJ Med. 2, e000651 (2023).
Nargesi, A. A. et al. Persistence on novel cardioprotective antihyperglycemic therapies in the United States. Am. J. Cardiol. 196, 89–98 (2023).
Schernthaner, G. et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc. Diabetol. 19, 185 (2020).
Levintow, S. N. et al. Lipid testing trends before and after hospitalization for myocardial infarction among adults in the United States, 2008–2019. Clin. Epidemiol. 14, 737–748 (2022).
Wang, W. T. et al. Lipid testing and statin dosing after acute myocardial infarction. J. Am. Heart Assoc. 7, e006460 (2018).
Reid, R. J. et al. Relationship between cardiovascular risk and lipid testing in one health care system: a retrospective cohort study. BMC Health Serv. Res. 15, 281 (2015).
Pivovarov, R., Albers, D. J., Hripcsak, G., Sepulveda, J. L. & Elhadad, N. Temporal trends of hemoglobin A1c testing. J. Am. Med. Inform. Assoc. 21, 1038–1044 (2014).
Driskell, O. J. et al. Inappropriate requesting of glycated hemoglobin (Hb A1c) is widespread: assessment of prevalence, impact of national guidance, and practice-to-practice variability. Clin. Chem. 58, 906–915 (2012).
Pearson, G. J. et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can. J. Cardiol. 37, 1129–1150 (2021).
Bittner, V. A. et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J. Am. Coll. Cardiol. 75, 133–144 (2020).
Schwartz, G. G. et al. Lipoprotein(a) and benefit of PCSK9 inhibition in patients with nominally controlled LDL cholesterol. J. Am. Coll. Cardiol. 78, 421–433 (2021).
O’Donoghue, M. L. et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 139, 1483–1492 (2019).
Novartis Pharmaceuticals A multicenter trial assessing the impact of lipoprotein(a) lowering with pelacarsen (TQJ230) on the progression of calcific aortic valve stenosis. Clinicaltrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT05646381 (2022).
Silence Therapeutics plc Evaluate SLN360 in participants with elevated lipoprotein(a) at high risk of atherosclerotic cardiovascular disease events. Clinicaltrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT05537571 (2022).
Eli Lilly and Company A study of LY3819469 in healthy participants. Clinicaltrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT04914546 (2021).
Eli Lilly and Company A study of LY3473329 in healthy participants. Clinicaltrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT04472676 (2020).
Joshi, P. H. et al. Heterogeneity of lipoprotein(a) levels among Hispanic or Latino individuals residing in the US. JAMA Cardiol. 8, 691–696 (2023).
Reeskamp, L. F. et al. Concordance of a high lipoprotein(a) concentration among relatives. JAMA Cardiol. 8, 1111–1118 (2023).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am. J. Epidemiol. 129, 687–702 (1989).
Friedman, G. D. et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol. 41, 1105–1116 (1988).
Bild, D. E. et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881 (2002).
Marcovina, S. M. et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin. Chem. 46, 1956–1967 (2000).
Ohira, T. et al. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 37, 1407–1412 (2006).
Gaubatz, J. W., Chari, M. V., Nava, M. L., Guyton, J. R. & Morrisett, J. D. Isolation and characterization of the two major apoproteins in human lipoprotein [a]. J. Lipid Res. 28, 69–79 (1986).
Khera, A. V. et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 129, 635–642 (2014).
Marcovina, S. M. et al. Lipoprotein[a] concentrations and apolipoprotein[a] phenotypes in Caucasians and African Americans. The CARDIA study. Arterioscler. Thromb. 13, 1037–1045 (1993).
Guan, W. et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 996–1001 (2015).
Goff, D. C. Jr et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49–S73 (2014).
UK Biobank. UK Biobank touch-screen questionnaire (2011); https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/TouchscreenQuestionsMainFinal.pdf
Elliott, P. & Peakman, T. C., UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 37, 234–244 (2008).
Fry, D., Almond, R., Moffat, S., Gordon, M. & Singh, P. UK Biobank Biomarker Project. Companion Document to Accompany Serum Biomarker Data (UK Biobank, 2019); https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf
Arik, S. Ö. & Pfister, T. TabNet: attentive interpretable tabular learning. In Proc. AAAI Conference on Artificial Intelligence Vol. 35, 6679–6687 (Association for the Advancement of Artificial Intelligence, 2021).
Chen, T. & Guestrin, C. XGBoost: a scalable tree boosting system. In Proc. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016).
Norgeot, B. et al. Minimum information about clinical artificial intelligence modeling: the MI-CLAIM checklist. Nat. Med. 26, 1320–1324 (2020).
Acknowledgements
The study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award R01HL167858 and K23HL153775 to R.K.) and the Doris Duke Charitable Foundation (under award 2022060 to R.K.). The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the paper; and decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
A.A. and R.K. conceived and designed the study. A.A. conducted statistical analyses. L.S.D., E.K.O., S.S., P.T., S.V., E.S., and R.K. interpreted the data. A.A. and L.S.D. wrote the first draft of the paper and prepared figures. E.K.O., S.S., P.T., S.V.S., E.S. and R.K. critically revised the paper. A.A. and S.V.S. made ARISE available online. R.K. acquired funding and supervised the study. All authors had full access to all data used in this study. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
R.K. is an associate editor at JAMA and receives research grant support, through Yale, from Bristol‐Myers Squibb, Novo Nordisk and BridgeBio. R.K. also receives support from the Blavatnik Foundation through the Blavatnik Fund for Innovation at Yale. R.K. is a cofounder of Ensight-AI, and R.K. and E.K.O. are cofounders of Evidence2Health, both representing precision health platforms to improve evidence-based cardiovascular care. E.K.O. has served as a consultant to Caristo Diagnostics Ltd. (Oxford, U.K.), unrelated to the current work. The other authors have no disclosures to report.
Peer review
Peer review information
Nature Cardiovascular Research thanks Dimitrios Doudesis, Erik Stroes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
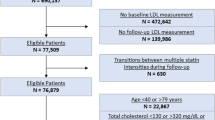
Extended Data Fig. 1 Lp(a) Assessment among the UK Biobank Participants.
Abbreviations: UKB, UK Biobank. The UK Biobank represents the largest cohort of individuals with protocolized Lp(a) assessment.
Extended Data Fig. 2 SHAP Values of ARISE’s Features Across UK Biobank Held-out Test Set, ARIC, CARDIA, and MESA Cohorts.
Abbreviations: ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; CARDIA, Coronary Artery Risk Development in Young Adults; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MESA, Multi-Ethnic Study of Atherosclerosis; SHAP, SHapley Additive exPlanations; UKB, UK Biobank. Across the held out test set and the external validation cohorts, we describe the impact of the 6 ARISE features on the model output probability.
Extended Data Fig. 3 ARISE’s Performance and Number Needed to Test Relative Reduction Across Demographic and Clinical Subgroups in the ARIC Cohort.
Abbreviations: ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; DM, diabetes mellitus; IHD, ischemic heart disease; NNT, number needed to test. The measure of center and the error bars represent the area under the receiver operating characteristic curve and the 95% confidence intervals.
Extended Data Fig. 4 ARISE’s Performance and Number Needed to Test Relative Reduction Across Demographic and Clinical Subgroups in the CARDIA Cohort.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; AUROC, area under the receiver operating characteristic curve; CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; DM, diabetes mellitus; IHD, ischemic heart disease; NNT, number needed to test. *Comprises of less than 10 participants with elevated Lp(a). The measure of center and the error bars represent the area under the receiver operating characteristic curve and the 95% confidence intervals.
Extended Data Fig. 5 ARISE’s Performance and Number Needed to Test Relative Reduction Across Demographic and Clinical Subgroups in the MESA Cohort.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; DM, diabetes mellitus; MESA, Multi-Ethnic Study of Atherosclerosis; NNT, number needed to test. The measure of center and the error bars represent the area under the receiver operating characteristic curve and the 95% confidence intervals.
Supplementary information
Supplementary Tables
Supplementary Tables 1–15.
Source data
Source Data Fig. 2
Raw data for receive operating characteristic curves.
Source Data Fig. 3
Raw odds ratios data for forest plots.
Source Data Fig. 4
Raw data for performance metrics across thresholds.
Source Data Fig. 5
Raw data for number of individuals, area under the receive operating characteristic curves and the relative reduction in number needed to treat.
Source Data Extended Data Fig. 3
Raw data for number of individuals, area under the receive operating characteristic curves and the relative reduction in number needed to treat.
Source Data Extended Data Fig. 4
Raw data for number of individuals, area under the receive operating characteristic curves and the relative reduction in number needed to treat.
Source Data Extended Data Fig. 5
Raw data for number of individuals, area under the receive operating characteristic curves and the relative reduction in number needed to treat.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aminorroaya, A., Dhingra, L.S., Oikonomou, E.K. et al. Development and multinational validation of an algorithmic strategy for high Lp(a) screening. Nat Cardiovasc Res 3, 558–566 (2024). https://doi.org/10.1038/s44161-024-00469-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00469-1