Abstract
During the processes of rice domestication and improvement, a trade-off effect between grain number and grain weight was a major obstacle for increasing yield. Here, we identify a critical gene COG1, encoding the transcription factor OsMADS17, with a 65-bp deletion in the 5′ untranslated region (5′ UTR) presented in cultivated rice increasing grain number and grain weight simultaneously through decreasing mRNA translation efficiency. OsMADS17 controls grain yield by regulating multiple genes and that the interaction with one of them, OsAP2-39, has been characterized. Besides, the expression of OsMADS17 is regulated by OsMADS1 directly. It indicates that OsMADS1-OsMADS17-OsAP2-39 participates in the regulatory network controlling grain yield, and downregulation of OsMADS17 or OsAP2-39 expression can further improve grain yield by simultaneously increasing grain number and grain weight. Our findings provide insights into understanding the molecular basis co-regulating rice yield-related traits, and offer a strategy for breeding higher-yielding rice varieties.
Similar content being viewed by others
Introduction
Crop domestication and improvement are outstanding events in human history. During the process of the wild rice (Oryza rufipogon Griff.) domesticated into cultivated rice (O. sativa L.), remarkable morphological transitions have occurred, such as superior plant architecture accompanied by decreased tiller number and tiller angle and higher grain yield resulted from increased both grain number and grain weight1,2,3,4,5,6,7,8,9,10,11,12,13,14 (Fig. 1a, b). After domestication, increasing grain yield remained to be the prime target in the process of rice improvement. Over the past decades, a number of key genes regulating grain number15,16,17,18,19,20,21,22,23,24,25 and grain weight26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 had been characterized, which largely contributed to the significant achievements in genetic improvement of rice grain yield. However, most of these genes only regulated one of the traits, or even negatively regulated the other one, which were known as the trade‐off effect between grain number and grain weight, and it restrained the greater achievements for improving rice grain yield. Therefore, how to overcome the trade‐off effect between grain number and grain weight and promoting them simultaneously is a challenging task left to be resolved in modern breeding programs.
a, b Phenotype of O. rufipogon (a) and O. sativa (b). Panicles and grains are shown in the lower corner. Scale bars, 5 cm for panicles, 1 cm for grains. c–e Main panicles (c), grain width (d), and grain length (e) of C418 and 8IL73 plants. Scale bars, 10 cm (c), 1 cm (d, e). f–h Comparison of grain number per main panicle (f), 1000-grain weight (g), and grain yield per plant (h) between C418 and 8IL73. Data were means ± standard deviation (s.d.) (n = 20 plants), comparisons are made by two-tailed Student’s t-test. Source data underlying Fig. 1f–h are provided as a Source Data file.
Genomic sequence variants arising in the gene-coding and regulatory regions are important genetic bases of trait variation. In terms of gene expression, these variants involve complex procedures, crucially including transcription and translation. Previous studies devoted most of their attention to changes in transcript levels for deciphering the mechanism of rice yield-related trait variation4,5,6,7,20,21,22,23,32,33,34,35,36,37,38,39. However, translation, including all the post-transcriptional processes, is more rarely mentioned, and sequence polymorphisms in the 5′ untranslated region (5′ UTR) for regulating rice grain yield through variation in translation efficiency without changes in transcript level or protein function are scarcely reported.
In the current study, we identify a wild rice introgression line 8IL73 with less and smaller grains compared with the recipient parent, a japonica variety, C418. Through genetic analysis and map-based cloning, we identify a quantitative trait locus CONTROL OF GRAIN YIELD 1 (COG1) encoding the transcript factor OsMADS17 simultaneously increasing both grain number and grain size through decreased translation efficiency caused by a 65-bp deletion in the 5′ UTR. OsMADS17 combined with the upstream gene OsMADS1 and the downstream gene OsAP2-39 participates in the regulatory network regulating rice grain yield, and the favorable variants of OsMADS1, OsMADS17, and OsAP2-39 can increase grain yield in rice improvement. Furthermore, the downregulation of OsMADS17 or OsAP2-39 expression in high-yielding rice varieties increases grain yield by further improving grain number and grain weight simultaneously. Our findings will provide not only insights into molecular mechanisms for increasing grain yield in rice, but also identify strategies for co-improvement of grain number and grain size.
Results
COG1 controls grain number and grain weight in rice
We found that a wild rice introgression line 8IL73, which harbors seven chromosomal segments from DXCWR, the donor common wild rice (O. rufipogon Griff.) parent from Dongxiang, Jiangxi Province, China (Supplementary Fig. 1), had less grains per main panicle (−14.9%), lower individual grain weight (−7.6%), and lower grain yield per plant (−17.8%) compared with the recipient parent, an elite japonica variety C418 (Fig. 1c–h and Supplementary Fig. 2). To identify the genetic factors responsible for co-regulating grain number and grain weight in DXCWR, we carried out a genetic linkage analysis using 203 F2 individuals derived from the cross between 8IL73 and C418, and detected a major quantitative trait locus (QTL) for grain number and grain weight located on the long arm of chromosome 4 (Supplementary Table 1 and Supplementary Fig. 3), which was named CONTROL OF GRAIN YIELD 1 (COG1). Through evaluating grain number per main panicle of each recombinant identified from 6123 F2 individuals, we fine-mapped COG1 to a 19.6-kb interval, which contained only one predicted gene (LOC_Os04g49150), encoding a MADS-box transcription factor OsMADS17 (Fig. 2a). Sequencing the transcript region of LOC_Os04g49150 revealed that there were ten single-nucleotide polymorphisms (SNPs) and six insertions/deletions (indels) between 8IL73 and C418 (Supplementary Fig. 4). Among these variations, a synonymous mutation (SNP 1) and a 65-bp insertion/deletion (Indel 1) existed in the coding region and the 5′ UTR, respectively (Supplementary Fig. 4). Reverse transcription quantitative PCR (RT-qPCR) analysis showed that the transcript levels of LOC_Os04g49150 were not significantly different between 8IL73 and C418 in developing young panicles (Supplementary Fig. 5a).
a COG1 was mapped onto the long arm of chromosome 4 in the 19.6-kb region. b–d Main panicles (b), grain width (c), and grain length (d) of C418 and C418CTP plants. e Relative expression levels of OsMADS17 in C418 and C418CTP plants (n = 3 replicates). f–h Comparison of grain number per main panicle (f), 1000-grain weight (g), and grain yield per plant (h) between C418 and C418CTP plants (n = 20 plants). i–k Same as (b–d) for 8IL73 and 8IL73RNAi-OsMADS17 plants. l Relative expression levels of OsMADS17 in 8IL73 and 8IL73RNAi-OsMADS17 plants (n = 3 replicates). m–o Same as (f–h) for 8IL73 and 8IL73RNAi-OsMADS17 plants (n = 20 plants). Scale bars, 10 cm (b, i), 1 cm (c, d, j, k). Data were means ± s.d., comparisons are made by two-tailed Student’s t-test. Source data underlying Fig. 2a, e–h, l–o are provided as a Source Data file.
As sequence comparisons and transcript level analysis did not provide enough evidence to estimate the functional allele of LOC_Os04g49150, we generated two reciprocal transgenic plants, C418CTP and 8IL73CTP. The C418CTP transgenic plants, which were generated by introducing the LOC_Os04g49150 gene from 8IL73 into C418 plants, showed a similar OsMADS17 expression level, but fewer grains per panicle, a lower grain weight, and grain yield than that of the C418 control plants (Fig. 2b–h and Supplementary Fig. 6). On the other hand, the 8IL73CTP transgenic plants, which were generated by introducing the LOC_Os04g49150 gene from C418 into 8IL73 plants, displayed no significant difference in grain yield-related traits compared with 8IL73 (Supplementary Fig. 7). To further verify the function of LOC_Os04g49150, we generated a OsMADS17 specific RNA interference (RNAi) vector (Supplementary Fig. 8a). Transgenic 8IL73 plants expressing the RNAi vector (8IL73RNAi-OsMADS17) exhibited a significant decrease in OsMADS17 expression levels and a significant increase in grain number, grain size, and grain yield compared with the controls (Fig. 2i–o and Supplementary Fig. 8b–j). Taken together, these results demonstrated that LOC_Os04g49150 was responsible for the COG1 gene, which functions as a simultaneous co-regulator for grain number and individual grain weight in rice.
A 65-bp deletion in the 5′ UTR of OsMADS17 increases grain yield
To identify the causal variation in the OsMADS17 gene between the two parental accessions, we used two genomic chimeric constructs to generate reciprocal, complementary transgenic plants C418CTP-WC (involving the 2.5-kb OsMADS17 promoter from 8IL73 fused with the OsMADS17 transcript region from C418 in a C418 background) and C418CTP-CW (involving the 2.5-kb OsMADS17 promoter from C418 fused with the OsMADS17 transcript region from 8IL73 in a C418 background) (Supplementary Fig. 9a). RT-qPCR and phenotypic analysis showed that no significant change in OsMADS17 expression levels appeared between C418 and either of the transgenic plants, C418CTP-CW or C418CTP-WC, but the C418CTP-CW transgenic plants had significantly fewer grains, significantly smaller grain size and lower grain yield than the C418 control, whereas the C418CTP-WC transgenic plants showed a similar phenotype to that of the C418 control (Fig. 3a–g, Supplementary Fig. 9b–j, and Supplementary Data 1). These results indicated that variants in the transcript region of OsMADS17 were responsible for the differences in grain number and individual grain weight between C418 and 8IL73.
a–c Main panicles (a), grain width (b), and grain length (c) of C418, C418CTP-WC, and C418CTP-CW plants. d Relative expression levels of OsMADS17 in C418, C418CTP-WC, and C418CTP-CW plants (n = 3 replicates). e–g Comparison of grain number per main panicle (e), 1000-grain weight (f), and grain yield per plant (g) among C418, C418CTP-WC, and C418CTP-CW plants (n = 20 plants). h Determination of specificity of Anti-OsMADS17 antibody. Purified recombinant MBP-OsMADS17 proteins were detected by anti-OsMADS17 and anti-MBP antibody (n = 3 replicates). i–m Comparison of OsMADS17 protein levels in young panicles of C418 and 8IL73 (i), C418 and C418CTP (j), C418, C418CTP-WC, and C418CTP-CW (k), 8IL73 and 8IL73RNAi-OsMADS17 (l), and 8IL73 and Mut-6 plants (m). HSP82 was used as the loading control (n = 3 replicates). n, o Dual-LUC reporter system for translation efficiency (TE) analysis. The 5′ UTR of OsMADS17 with De65-bp attenuated TE (n), and the fragment F3 with 22-bp at the end of the 5′ region was critical for the 65-bp fragment increasing TE (o) (n = 3 replicates). p–r Same as (a) to (c) for 8IL73 and Mut-6 plants. s Relative expression levels of OsMADS17 in 8IL73 and Mut-6 plants (n = 3 replicates). t–v Same as (e–g) for 8IL73 and Mut-6 plants (n = 20 plants). w The frequency of genotypes OsMADS17De65-bp and OsMADS17In65-bp in cultivated rice. x The geographical distribution of cultivars with genotype OsMADS17De65-bp in areas of Asia. Scale bars, 10 cm (a, p), 1 cm (b, c, q, r). In d–g, o, the statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, different letters represent significant differences (P < 0.05), and the exact P values were provided in Supplementary Data 1, 2. In i–n, s–v, comparisons are made by two-tailed Student’s t-test. Data were means ± s.d. Source data underlying Fig. 3d–o, s–x are provided as a Source Data file.
To reveal the expression pattern of OsMADS17, we carried out RT-qPCR and RNA in-situ hybridization analysis, which showed that OsMADS17 was predominately expressed in the developing panicle and in panicle branch primordia, but that there was no significant difference in OsMADS17 transcript levels between 8IL73 and C418 (Supplementary Fig. 5a, c–e). As no differences were detected in neither the transcript expression levels of the OsMADS17 gene nor the amino acid sequence of OsMADS17 between 8IL73 and C418, we carried out western blot assays using the specific antibody Anti-OsMADS17 (Fig. 3h) and found that C418 contained lower OsMADS17 protein levels than that of 8IL73 in young panicles and other tissues, such as root, tiller base, and node (Fig. 3i and Supplementary Fig. 5b). Further analysis for OsMADS17 protein levels in transgenic plants showed that C418CTP and C418CTP-CW contained higher OsMADS17 protein levels than in C418 plants (Fig. 3j, k), although no significant change in OsMADS17 protein levels were apparent between C418CTP-WC and C418 plants (Fig. 3k). Correspondingly, the 8IL73RNAi-OsMADS17 transgenic plants had lower OsMADS17 protein accumulation than did the control (Fig. 3l). Therefore, we speculated that the 65-bp indel (for convenience, the 65-bp insertion is hereafter termed In65-bp, and the deletion of 65-bp is termed De65-bp) is probably involved in regulating the process of OsMADS17 translation and causing the function change of OsMADS17.
To test this hypothesis, we conducted transient expression assays, using the dual-luciferase reporter system. The constructs with 5′ UTRDe65-bp of OsMADS17 generated lower LUC/REN activity levels and lower translation efficiencies (TE), but no significant change in LUC/REN mRNA levels compared with 5′ UTRIn65-bp (Fig. 3n). Further analysis indicated that the fragments F3 (22-bp of the 5′ region of the 65-bp indel), F5 (32-bp at the end of 5′ region in the 65-bp, including the fragment F3), and the full length of 65-bp sequence promoted TE significantly compared with the other fragments within the 65-bp sequence. These results confirmed that the In65-bp in the 5′ UTR of the OsMADS17 gene promoted TE significantly, and that the 22-bp sequence at the end of the 5′ region of the 65-bp was crucial for increasing translation (Fig. 3o and Supplementary Data. 2).
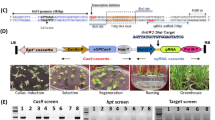
To further assess the function of the 65-bp sequence for increasing TE of the OsMADS17 gene in rice plants, we selected the F3 fragment, which was located at the interval of PAM1 (GGG) and PAM2 (TGG) as the target for genome editing by the CRISPR/Cas9 system in 8IL73 plants (Supplementary Fig. 10a). Among the six types of transgenic plants, only the Mut-6 plants with the 34-bp deletion containing the F3 fragment exhibited significantly increased grain number, grain weight, and grain yield compared to 8IL73, accompanied by decreased TE and accumulation levels of OsMADS17 protein, but no significant differences in mRNA levels (Fig. 3m, p–v and Supplementary Fig. 10b–j). Taken together, the De65-bp in the 5′ UTR of the OsMADS17 gene significantly attenuated TE and resulted in increased grain number, grain weight, and grain yield.
To explore the distribution of the 65-bp indel in rice populations, we screened 82 accessions of wild rice and 169 varieties of cultivated rice (including 79 indica and 90 japonica varieties) for the 65-bp indel in the 5′ UTR of OsMADS17 and found that all 82 accessions of wild rice harbored the 65-bp insertion (Supplementary Data 3), whereas 111 of the cultivars contained the In65-bp, with the rest of 58 cultivars carrying the De65-bp; no other allele was found (Supplementary Fig. 11a, b). We further analyzed the distribution of the 65-bp indel in 2775 cultivated rice accessions from the 3 K RG (RFGB v2.0 database, https://www.rmbreeding.cn/). The distribution study showed that the frequency of cultivars with the genotype OsMADS17De65-bp in japonica, indica, and aus rice was 44.2, 14.8, and 26.0%, respectively (Fig. 3w), with the japonica varieties with OsMADS17De65-bp being distributed mainly in East Asia, whereas the indica varieties with OsMADS17De65-bp were mainly distributed in Southeast Asia (Fig. 3x). These results confirmed that the 65-bp deletion in the 5′ UTR of the OsMADS17 gene originated from the mutation in cultivated rice.
To investigate the relationship between the 65-bp indel and grain number or grain weight, we analyzed 169 cultivated rice accessions with respect to the phenotype and the genotype at the OsMADS17 locus. Analysis showed that the 58 cultivars with the genotype OsMADS17De65-bp displayed significantly higher individual grain weight than the 111 cultivars with the genotype OsMADS17In65-bp, whereas no significant difference in grain number was observed between the two groups (Supplementary Fig. 11a, b). Further analysis of 1591 cultivars from 3 K RG showed that 403 cultivars containing the genotype OsMADS17De65-bp exhibited significantly higher grain weight than the 1188 cultivars with the genotype OsMADS17In65-bp (Supplementary Fig. 11c). These results indicated that the 65-bp deletion was associated with larger grains in cultivated rice.
OsMADS17 targets OsAP2-39 to regulate grain yield
In order to identify the downstream genes regulated by OsMADS17, we first performed RNA-sequencing analysis using young panicles from C418, 8IL73, and 8IL73RNAi-OsMADS17 plants. As a result, 536 genes were shown to be significantly differentially expressed between C418 and 8IL73, whereas 953 differentially expressed genes (DEGs) were detected between 8IL73RNAi-OsMADS17 and 8IL73 (Supplementary Fig. 12a). We identified 355 overlapping DEGs between the two comparative groups for analysis with respect to the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. It showed that OsMADS17 participated in multiple regulatory pathways, such as regulation of transcription, gene expression, and plant hormone signal transduction (Supplementary Fig. 12b–d). Among the 355 overlapping DEGs, we found 11 genes of the APETALA2 (AP2) family (Fig. 4a), which play critical roles during the processes of floral organ development. Among the 11 genes, OsAP2-39 (LOC_Os04g52090) expression was proved to be negatively correlated with rice grain number and yield in a previous study43, and RT-qPCR analysis also further confirmed significant differences in OsAP2-39 expression in C418, 8IL73, and 8IL73RNAi-OsMADS17 plants (Fig. 4b). To explore the interaction between OsMADS17 and OsAP2-39, we analyzed the promoter region of OsAP2-39, finding that CArG motifs located at ~2-kb upstream of the start codon (ATG) were putative binding sites (PBS) for MADS-box transcription factors. Yeast one-hybrid (Y1H) assays and Electrophoretic Mobility Shift Assays (EMSA) proved that OsMADS17 could bind to the PBS, and the transient expression system in rice protoplasts showed that OsMADS17 promoted the activation of the 2.4-kb promoter of OsAP2-39 (Fig. 4c–e). These results confirmed that OsMADS17 regulated the expression levels of OsAP2-39 positively by interacting physically with the promoter region.
a Differentially expressed AP2/ERF family genes revealed by the RNA-sequencing analysis. b Validation for the expression levels of OsAP2-39 by RT-qPCR (n = 3 replicates). c, d Yeast one-hybrid (c) and EMSA assays (d) proved OsMADS17 bound to the promoter region of OsAP2-39 (n = 3 replicates). e Dual-LUC reporter assays indicated OsMADS17 promoted activation of the promoter of OsAP2-39. Control, cotransfected with reporter construct and an empty effector construct (set to 1) (n = 3 replicates). f–h Main panicles (f), grain width (g), and grain length (h) of C418 and C418OE-OsAP2-39 plants. i Comparison of grain number per main panicle, 1000-grain weight, and grain yield per plant between C418 and C418OE-OsAP2-39 plants (n = 20 plants). j–m Same as (f–i) for C418 and C418RNAi-OsAP2-39 plants (n = 20 plants). n Association test between 1000-grain weight and variants (with minor allele frequency >0.05) in the 3-kb promoter region of OsAP2-39, and the variants were accepted by the standard of P < 0.001. o Cultivars with haplotype OsAP2-39T (with genotype T at S2 position) showed bigger grain size than the others. No. of cvs., number of cultivars. p The frequency of genotypes OsAP2-39T and OsAP2-39C in cultivated rice. q The frequency of favorable genotypes in cultivated rice. r Geographical distribution of favorable genotypes in Asia areas. Scale bars, 10 cm (f, j), 1 cm (g, h, k, l). Data for b, e, i, m are means ± s.d., comparisons are made by two-tailed Student’s t-test. Source data underlying Fig. 4a–e, i, m–r are provided as a Source Data file.
To determine whether the OsAP2-39 gene was responsible for rice grain yield regulation, we constructed the OsAP2-39 overexpressed (OE-OsAP2-39) and OsAP2-39 specific RNA interference (RNAi-OsAP2-39) vectors (Supplementary Fig. 13a) to generate C418OE-OsAP2-39 and C418RNAi-OsAP2-39 transgenic plants, respectively. The C418OE-OsAP2-39 overexpressed plants produced fewer grains, smaller grain size, and lower grain yield (Fig. 4f–i and Supplementary Fig. 13b–k), whereas the C418RNAi-OsAP2-39 RNAi plants resulted in an increase in grain number, grain size, and grain yield (Fig. 4j–m and Supplementary Fig. 13l–u) compared with C418 plants. To explore the relationship between the grain yield-related traits and nucleotide polymorphisms at the OsAP2-39 locus, we searched the 3 K RG database with an association test between 1000-grain weight (TGW) and sequence variations. Among all of the variants in the 3-kb promoter region, one SNP (S2, T > C, 2256-bp upstream of the start codon (ATG)) showed the strongest signal correlating with TGW (Fig. 4n). Haplotype analysis also showed that cultivars with the OsAP2-39T haplotype exhibited heavier TGW than those with OsAP2-39C (Fig. 4o and Supplementary Fig. 14a, b). In addition, RT-qPCR analysis in young panicles with a length of 0–0.5 cm revealed that cultivars with OsAP2-39T showed lower expression levels of OsAP2-39 than did cultivars with OsAP2-39C (Supplementary Fig. 14c). These results indicated that OsAP2-39 regulated rice grain number, grain weight, and grain yield negatively.
To gain an insight into the distribution of the OsAP2-39T/C genotype in different rice populations, we screened the 82 accessions of wild rice, finding all of them contained the genotype OsAP2-39C (Supplementary Data 3). In the 2,775 cultivated rice accessions from rice 3 K RG, the frequency of cultivars with OsAP2-39T in japonica, indica, and aus rice was 11.6, 6.6, and 4.1%, respectively (Fig. 4p). In addition, the combination of genotype OsAP2-39T with genotype OsMADS17De65-bp occurred in cultivated rice at a much lower frequency than those having only OsAP2-39T or OsMADS17De65-bp (Fig. 4q). Furthermore, the OsAP2-39T genotype mainly appeared in East Asia and Southeast Asia in both indica and japonica rice populations, whereas the combination of OsAP2-39T with OsMADS17De65-bp appeared only in indica rice, being mainly distributed in Southeast Asia (Fig. 4r). Taken together, OsAP2-39T is a rare mutation in cultivated rice, and the favorable variation of OsAP2-39T and OsMADS17De-65bp combined together only existed in indica rice.
OsMADS17 expression is regulated directly by OsMADS1
To identify the upstream genes of OsMADS17, we scanned the promoter region and found two CArG motifs, one of which could probably interact with OsMADS144, which is a MADS-box transcription factor involved in grain length regulation30,31. Y1H (Fig. 5a) and EMSA (Fig. 5b) assays confirmed that OsMADS1 could bind to the promoter region of OsMADS17, and the transient expression system in rice protoplasts showed that OsMADS1 promoted the activation of the promoter of OsMADS17 (Fig. 5c). In addition, downregulated expression of OsMADS1 was accompanied by decreased expression of OsMADS17 (Fig. 5d). This evidence indicated that OsMADS1 regulated the expression of OsMADS17 both positively and directly.
a, b Yeast one-hybrid (a) and EMSA (b) assays proved that OsMADS1 is bound to the promoter region of OsMADS17. c Dual-LUC reporter assays indicated that OsMADS1 promoted the activation of the promoter of OsMADS17 in both C418 and 8IL73 plants. Control, cotransfected with reporter construct and an empty effector construct (set to 1) (n = 3 replicates). d–f Relative expression levels of OsMADS1, OsMADS17, and OsAP2-39 in young panicles (0–0.5 cm) of C418, C418RNAi-OsMADS1, C418RNAi-OsMADS17, and C418RNAi-OsAP2-39 plants (n = 3 replicates). g Cultivars with haplotype OsMADS1AS (with genotype A at S9 position) showed bigger grain size than the others. No. of cvs., number of cultivars. h The frequency of genotypes OsMADS1AS and OsMADS1NS in cultivated rice accessions. i The frequency of different combinations of favorable genotypes in cultivars. j Geographical distribution of favorable genotypes in Asia areas. k OsMADS1, OsMADS17, and OsAP2-39 participated in the regulatory network controlling rice grain yield. Data for c–f are means ± s.d. and comparisons are made by two-tailed Student’s t-test. Source data underlying Fig. 5a–j are provided as a Source Data file.
To identify the mechanism of OsMADS1 in controlling grain yield, we generated RNAi transgenic plants of OsMADS1 in the C418 background (C418RNAi-OsMADS1). Analysis showed that the C418RNAi-OsMADS1 plants generated significantly longer grains, but there was no significant difference in grain number compared with the control (Supplementary Fig. 15). As the C418RNAi-OsMADS1 plants formed abnormal grains with hulls which were not closed, they showed a decrease in TGW and yield compared with C418 (Supplementary Fig. 15). Recently, it has been reported that alternative splicing (AS) of the OsMADS1 transcript was associated with increased grain length and grain yield compared with native splicing (NS, which was accordant with that in the japonica rice cultivar Nipponbare)30,31. Haplotype analysis also showed that varieties with the OsMADS1AS haplotype exhibiting larger grains than those with the OsMADS1NS haplotype (Fig. 5g). RT-qPCR and western blot analysis showed no significant difference in OsMADS1, OsMADS17, and OsAP2-39 transcript levels, as well as OsMADS17 protein levels between NIL-OsMADS1AS and NIL-OsMADS1NS plants (Supplementary Fig. 16). However, the expression levels of OsMADS1, OsMADS17, and OsAP2-39 were significantly lower in the C418RNAi-OsMADS1 plants compared with the control (Fig. 5d). These results suggested that OsMADS1 might regulate grain length by controlling both OsMADS17 and OsAP2-39 expression.
To explore the genetic interactions among OsMADS1, OsMADS17, and OsAP2-39, we first analyzed the functions of different alleles of these genes with respect to the regulation of grain yield-related traits, based on the genotypes and phenotypes of accessions from the 3 K RG. We found that cultivars carrying the genotypes OsMADS1AS, OsMADS17De65-bp, or OsAP2-39T showed higher individual grain weight than those with the genotypes OsMADS1NS, OsMADS17In65-bp, or OsAP2-39C, respectively (Supplementary Figs. 11b, c, 17a). The individual grain weight of the cultivars carrying both OsMADS1AS and OsAP2-39T was significantly higher than that in cultivars containing only OsMADS1AS or OsAP2-39T (Supplementary Fig. 17b). In addition, cultivars with the genotype OsMADS17De65-bp and OsAP2-39T produced larger grains than those carrying only OsMADS17De65-bp or OsAP2-39T (Supplementary Fig. 17c). These results suggested that all of the genes OsMADS1, OsMADS17, and OsAP2-39 were involved into regulating individual grain weight. To further elucidate the genetic interaction between OsMADS17 and OsAP2-39, we generated the null single mutants osmads17 and osap2-39, and the double mutant osmads17/osap2-39 in the ZH17 background using the CRISPR/Cas9 system (Supplementary Fig. 18a). Phenotypic analysis showed that the single mutants osmads17 or osap2-39 exhibited significantly increased individual grain weight and grain yield compared with the control (Supplementary Fig. 18b–o and Supplementary Data 4), while the double mutant osmads17/osap2-39 further increased grain weight and grain yield compared with the single mutants osmads17 or osap2-39 (Supplementary Fig. 18b–o and Supplementary Data 4), indicating that OsMADS17 and OsAP2-39 have additive effects on grain yield regulation. To further investigate the regulatory relationship among OsMADS1, OsMADS17, and OsAP2-39, we analyzed the expression levels of these genes in RNAi transgenic plants in the C418 background, finding that the C418RNAi-OsMADS17 plants showed decreased expression of OsMADS17 and OsAP2-39, but no obvious change in OsMADS1 expression compared with the control (Fig. 5e). In the C418RNAi-OsAP2-39 plants, unlike the interfered gene OsAP2-39, there was no significant decrease in expression of OsMADS1 and OsMADS17 relative to the control (Fig. 5f). Taken together, these findings showed that OsMADS1, OsMADS17, and OsAP2-39 participated in the regulatory network controlling grain yield (Fig. 5k).
To reveal the distribution of different genotypes for OsMADS1, OsMADS17, and OsAP2-39 in rice populations. We studied the 82 accessions of wild rice, discovering that all of them possessed the genotype OsMADS1NS (Supplementary Data 3), whereas the genotype OsMADS1AS almost appeared only in japonica rice, with a frequency of 56.0% (Fig. 5h), which meant that the OsMADS1AS gene originated from japonica rice. In addition, the frequencies of combinations of OsMADS1AS with OsMADS17De65-bp or OsAP2-39T in cultivated rice accessions were both much lower than the frequency of accessions having only OsMADS1AS, and only 0.2% of cultivars had the genotype with OsMADS1AS, OsMADS17De65-bp, and OsAP2-39T simultaneously (Fig. 5i). Furthermore, the geographical distribution of OsMADS1AS partially overlapped with the distribution of OsAP2-39T in Southeast Asia, whereas OsMADS1AS and OsMADS17De65-bp overlapped only at a very low frequency (Fig. 5j). Taken together, these findings indicated that OsMAD1AS and OsMADS17De65-bp were distributed independently in cultivated rice, and that OsAP2-39T and OsMADS1AS appeared simultaneously merely in japonica rice.
OsMADS17 or OsAP2-39 downregulation may increase grain yield in rice breeding
As OsMADS17 is a negative regulator controlling grain yield in rice, reducing its expression level perhaps could contribute to increasing rice grain yield. The above results had demonstrated that downregulated expression levels of OsMADS17 with In65-bp in 8IL73 plants could increase grain yield (Fig. 2i–o, Supplementary Fig. 8b–j), which meant great prospect for improving grain yield of the 75.9% cultivars harboring the 65-bp insertion of OsMADS17 (Fig. 4q). To further explore the potential of OsMADS17 increasing grain yield in the remaining of 24.1% cultivated rice with the beneficial 65-bp deletion (Fig. 4q), we generated the transgenic plants C418RNAi-OsMADS17 with downregulated OsMADS17 expression levels, which showed lower levels of OsMADS17 protein accumulation, higher grain number, bigger grain size, and higher grain yield compared with the controls (Fig. 6a–i and Supplementary Fig. 19). Field trials showed that the C418RNAi-OsMADS17 transgenic plants increased grain yield by 6.5% in Beijing (40.1°N, 116.2°E), northern China, and 12.3% in Hainan Province (18.3°N, 109.2°E), southern China, compared with the control plants (Fig. 6j, k). It indicated that reducing the expression levels of OsMADS17 in cultivated rice was desirable to improve grain yield.
a–d The whole plants (a), main panicles (b), grain width (c), and grain length (d) of C418 and C418RNAi-OsMADS17 plants. e Relative expression levels of OsMADS17 in C418 and C418RNAi-OsMADS17 plants (n = 3 replicates). f Comparison of OsMADS17 protein levels in young panicles between C418 and C418RNAi-OsMADS17 plants by western blot assays. HSP82 was used as the loading control (n = 3 replicates). g–i Comparison of grain number per main panicle (g), 1000-grain weight (h), and grain yield (i) between C418 and C418RNAi-OsMADS17 plants (n = 20 plants). j, k Comparison of grain yield in the field trials between C418 and C418RNAi-OsMADS17 plants (n = 3 replicates). 2019, Beijing, China (j) and 2020, Hainan Province, China (k). l Comparison of grain yield in the field trials between C418 and C418RNAi-OsAP2-39 plants (n = 3 replicates). 2021, Beijing, China. Scale bars, 25 cm (a), 10 cm (b), 1 cm (c, d). Data were means ± s.d. and comparisons are made by two-tailed Student’s t-test. Source data underlying Fig. 6e–l are provided as a Source Data file.
Additionally, only 8.2% of cultivated rice carried a beneficial variation of genotype OsAP2-39T (Fig. 4q), which means that reducing the expression levels of OsAP2-39 in cultivated rice to improve rice grain yield has tremendous potential. In C418RNAi-OsAP2-39 transgenic plants, the grain number per main panicle, individual grain weight, and grain yield increased by 8.6, 6.2, and 15.6% compared with the control, respectively (Fig. 4m). Field trials showed that the C418RNAi-OsAP2-39 transgenic plants increased grain yield by 7.3% in Beijing, compared with the control plants (Fig. 6l). These results indicated that downregulating expression levels of OsAP2-39 were feasible and achievable strategies for further increasing grain yield in cultivated rice.
Discussion
Crop domestication and improvement has been an important mission in the activities of human society for more than 10,000 years45. Understanding the mechanisms that can increase the grain yield of crop species will guide future breeding efforts, accelerating the development of new improved varieties. To date, a series of the key genes in rice controlling grain number and/or individual grain weight and associated with domestication and plant breeding, such as PROG11,2, FZP6,7, OsKRN214, Gn1a15, IPA120,21, GS327, and GW833,34, have been identified. However, the molecular mechanisms for simultaneously co-promoting grain number and individual grain weight in crop domestication and improvement remain largely unknown.
The trade-off effect between grain number and grain weight, in which, for example, increased grain number per panicle is associated with smaller grains, limits any increases in grain yield. Overcoming this trade-off effect has been a tough task for rice improvement. Here, we found that a 65-bp deletion in the 5′ UTR of the OsMADS17 gene, decreasing translation efficiency or downregulated transcript levels of OsMADS17, could increase grain number and individual grain weight simultaneously. As a transcription factor, OsMADS17 probably participated in the regulation of the expression of multiple genes. For instance, the downstream gene OsAP2-39, which was positively and directly regulated by OsMADS17, also simultaneously controlled grain number and individual grain weight. Furthermore, the RNA-seq results revealed that the genes OsGSR1/GW6/OsGASR738,39,40, OsCCA146, OsABCG1847, OsCEP6.148, GIF149, and OsMKKK7050, which regulated grain number and/or grain weight, were differentially expressed between C418 and 8IL73 (Supplementary Table 2). It is worth mentioning that OsABCG18 regulates grain number by affecting shootward transport of root-derived cytokinins47, and OsGSR1/GW6/OsGASR7 regulates grain size by controlling brassinosteroid (BR) and gibberellin (GA) signaling38,39,40. Therefore, we speculated that OsMADS17 was probably involved in regulating the expression levels of these genes to overcome the trade-off effect between grain number and grain weight. However, the detailed molecular mechanisms still remain to be elucidated.
Gene expression levels, which are normally presented as mRNA measurements51, are focused on deciphering the mechanism of phenotypic variation in rice. However, the mRNA levels cannot completely reflect the protein levels, as sophisticated multistep processes exist from pre-mRNA to mature protein generated, during which translation is critical for the determination of protein accumulation, affecting rice growth and development profoundly. Nevertheless, the mechanisms behind variation in gene expression, caused by translational changes, in the regulation of rice grain yield-related traits are seldom elucidated. In the current study, the De65-bp in the 5′ UTR of OsMADS17 was shown to decrease translation efficiency leading to altered grain yield resulting from simultaneous increases in grain number and individual grain weight, indicating that diversity of translation levels could also lead to dramatic phenotypic variation in rice, as well as changes in protein function and mRNA transcript levels. Furthermore, the 65-bp fragment in the 5′ UTR of the OsMADS17 gene might be used as a translation enhancer, increasing endogenous protein levels to regulate these important traits in rice plants. Therefore, these findings provide insight into revealing the mechanism of variation in grain yield-related traits from the perspective of mRNA translation.
In this present study, we demonstrated that OsMADS1-OsMADS17-OsAP2-39 participated in the regulatory network controlling grain number and grain weight in rice. On the one hand, members within the regulatory network could boost grain yield individually by altering protein function (OsMADS1), changing translation (OsMADS17), or transcription (OsAP2-39) processes. On the other hand, the genotype OsAP2-39T could further increase grain weight when in combination with the genotype OsMADS1AS or OsMADS17De65-bp, which meant that diverse combinations of the favorable alleles among the regulatory network could bring about additional avenues for achieving increased grain yield. In addition, downregulating the expression of OsMADS17 or OsAP2-39 in the regulatory network could co-enhance grain number and grain weight in rice. These findings not only helped to dissect the mechanism for increasing grain number and grain weight simultaneously for rice improvement, but also provided strategies for co-promoting these two key components of grain yield in rice breeding programs.
Methods
Plant materials and growing conditions
The DXCWR-derived introgression line 8IL73 and the recipient parent C418, an elite japonica variety, were used for phenotypic evaluation, fine mapping, expression pattern analysis, and the generation of transgenic plants. The japonica cultivar ZH17 was used for generating null single mutants osmads17 or osap2-39, and the double mutant osmads17/osap2-39. Materials for field trials of grain yield and associated yield components were planted in a randomized complete block design with three replicates (300 plants for each replication), and the planting density was one plant per 15 cm × 25 cm hill. Plant materials were grown under standard agronomic conditions (fertilizers, crop protection chemicals, etc.) at the experimental stations of China Agricultural University in Beijing and Hainan Province, China.
Trait measurements
Yield-related traits were measured when the plants were mature. Panicle length, primary branch number, grain number on the primary branch, secondary branch number, grain number on the secondary branch, grain length, grain width, 1000-grain weight, grain number per main panicle, and grain yield per plant were recorded. Yield per plant was scored as the total weight of filled grains from the entire plant. Grain length, grain width, and 1000-grain weight were measured using an automatic seed counting and analyzing instrument (Model SC-G, Wanshen).
Primers
The primers used in this study are listed in Supplementary Data 5.
Genetic analysis and positional cloning
The grain number on the main panicle and 1000-grain weight were evaluated for QTL analysis using 203 F2 individuals derived from the cross between C418 and 8IL73. Fine mapping was carried out using 6123 F2 individuals.
Transgene construct preparation and rice transformation
A total of four different combinations of constructs were used to generate reciprocal transgenic plants (C418CTP, 8IL73CTP, C418CTP-WC, and C418CTP-CW) in this study. Target fragments described in the Results were inserted into the vector of pCAMBIA1300 to form complementary constructs. The RNAi constructs, which harbored a hairpin structure with 301-bp (RNAi for OsMADS1), 308-bp (RNAi for OsMADS17), and 348-bp (RNAi for OsAP2-39) inverted repeat cDNA sequences, were inserted into the vector pTCK303/JL1460. The specificity of the RNAi vectors was confirmed by testing the expression levels of 12 MADS family genes or 12 AP2 family genes. The coding sequence of OsAP2-39 was amplified and inserted into the binary vector pCAMBIA1301 to generate the overexpression construct. To generate fragment deletions within the 65-bp sequence in 5′ UTR of OsMADS17, a robust CRISPR/Cas9 system was used for construct generation52. To acquire the null mutants for osmads17, osap2-39, and the double mutant osmads17/osap2-39, another CRISPR/Cas9 system was used53. All constructs were introduced into Agrobacterium tumefaciens strain EHA105 and subsequently transformed into C418, 8IL73, and ZH17 through Agrobacterium-mediated transformation for various purposes22. Target fragments of the hygromycin gene and the OsMADS17 gene containing the 65-bp indel were amplified using the primers HYG and InDel-65bp, respectively, listed in Supplementary Data 5 to identify the positive transgenic plants. Transgenic plants of the T2 generation were used for phenotypic evaluation.
RNA extraction and RT-qPCR
Total RNAs were extracted from the various tissues using TRIzol reagent (Invitrogen Life Technologies) and purified with a purification kit (Qiagen). First-strand cDNA was synthesized from 2.5 μg of total RNA in a 20 μL reaction volume using 2 μL oligo(dT)18 primer (Takara) and 1 μL SuperScript® III Reverse Transcriptase (Invitrogen). RT-qPCR was carried out using a CFX96 Real-Time System (Bio-Rad) or StepOnePlus (Applied Biosystems), with the rice Ubiquitin gene used as an internal control. cDNA (30 × dilutions) was amplified using the SsoFast EvaGreen Supermix (Bio-Rad). The relative quantification method (2−ΔΔCT) was used to evaluate the quantitative variation of expression (relative to Ubiquitin expression), and each set of experiments were repeated independently three times54.
RNA in-situ hybridization experiment
Young panicles of accessions C418 and 8IL73 were fixed in FAA solution (50 ml of ethanol, 5 ml of acetic acid, 10 ml of 37% formaldehyde, and 35 ml of DEPC-H2O) at 4 °C overnight, followed by a series of dehydrations using ethanol from 50 to 100% and infiltration using xylene from 50 to 100%, and were then embedded in paraffin (Paraplast Plus, Fisher Scientific). The tissues were sliced into approximately 8-μm-thick sections with a microtome (Leica RM2145), and the sections were placed on RNase-free glass slides. The 308-bp coding fragment of the OsMADS17 gene was amplified from cDNA to form probes. Digoxigenin-labeled sense and antisense RNA probes were generated using a DIG Northern Starter Kit (Roche), according to the manufacturer’s instructions. Slides were observed under a light microscope (Leica DMR) and photographed with a micro color camera (Apogee Instruments)55.
Western blot analysis
For this assay, young panicles (~1 cm) were collected and grounded into powder in liquid nitrogen and then protein extraction buffer was added (10% glycerol, 5 mM MgCl2, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% IGEPAL CA-630 (Sigma), 0.5 mM DTT (Sigma), 1 mM PMSF (Sigma) and 1× complete protease inhibitor cocktail (Roche)) for total protein extraction. The coding sequences of OsMADS17 were amplified and inserted into the pMAL-c5x vector to generate recombinant constructs for expressing the MBP (Maltose Binding Protein) fused protein. The recombinant constructs were transformed into the Escherichia coli Rosetta (DE3) strain, which were cultured at 37 °C (220 rpm) until the OD600 of the cell culture was 0.5. The MBP fused protein was then induced at 16 °C (180 rpm) with 0.5 mM of isopropyl β-d-1-thiogalactopyranoside (IPTG) for 16 h. E. coli cells were lysed by an ultrasonic cell crusher (Qsonica, Q700) and centrifuged. The supernatant containing MBP-OsMADS17 protein was affinity-purified using amylose resin (New England Biolabs). The proteins were boiled and electrophoretically separated by 8% (w/v) SDS-PAGE gel electrophoresis, and the polypeptides were transferred to a nitrocellulose membrane (GE Healthcare). A polyclonal antibody for OsMADS17 was raised in rabbits using a specific fragment consisting of OsMADS17 amino acids (C-NKINRQVTFSKRRN) by AbMART (http://www.ab-mart.com.cn/). Specific antibody to OsMADS17 (1:2,000 dilution), antibody to MBP (Cat# AbM59007-3-PU, Beijing Protein Innovation (BPI), 1:1,000 dilution) and HSP82 (Cat# AbM51099-31-PU, Beijing Protein Innovation (BPI), 1:5,000 dilution) were used for detection. HSP82 and OsMADS17 bands were analyzed with the Image J program (ImageJ 1.48, https://imagej.net/ij/) to calculate the relative protein levels according to the software instruction manual.
Transient expression assays for translation efficiency analysis
For translation efficiency examination, the 5′ UTR of OsMADS17 in C418, 8IL73, and Mut6 were amplified and inserted upstream of the luciferase (LUC) coding region in an expression cassette driven by the CaMV 35S promoter to form the reporter. A second expression cassette of Renilla reniformis luciferase (REN) as an internal vector control was also driven by the 35S promoter56. Rice protoplasts were isolated from 10-d-old seedlings grown on MS medium under dark conditions6. Constructs were transformed (10 μg plasmid) into rice protoplasts for transient expression. After incubating for 10 and 16 h at 28 °C in the dark, total RNA was extracted to generate cDNA for RT-qPCR analysis and the relative luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega), respectively. LUC/REN mRNA levels and relative LUC/REN activity conferred by each construct were normalized to those of the controls57. To detect the Firefly Luciferase (LUC) and Renilla Luciferase (REN) protein, antibodies Anti-LUC (Cat# T55401, AbMART, 1:1,000 dilution) and Anti-REN (Cat# T55403, AbMART, 1:1,000 dilution) were used for western blot analysis as described in the part of Western blot analysis. Translation efficiency (TE) is the rate of mRNA translated into proteins within cells. The relative TE was calculated as the ratio of relative LUC/REN activity to relative mRNA levels of LUC/REN in rice protoplast (TE=Relative LUC/REN activity/relative mRNA levels of LUC/REN).
RNA-seq analysis
Total RNA was isolated from the young panicles (0.2–0.4 cm) of C418, 8IL73, and 8IL73RNAi-OsMADS17, using TRIzol reagent (Invitrogen) and purified using an RNeasy Plant Mini Kit (Qiagen). Each sample contained 20 plants, with three biological replicates, and were used for paired-end library construction. Sequencing was performed on an Illumina platform at Novogene company, China (https://cn.novogene.com/). HTSeq v0.9.1 was used to count the reads numbers mapped to each gene. The DESeq R package (1.18.0), which provided statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution, was used for analyzing the differentially expressed genes. The resulting p values were adjusted by Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted p value (q value) <0.01 found by DESeq were assigned as differentially expressed. The functional category analysis of the DEGs was performed using agriGO and KEGG58,59.
Yeast one-hybrid (Y1H) assays
The coding sequence of OsMADS1 and OsMADS17 were subcloned into the pGADT7 vector to generate prey constructs. The fragments with binding sites corresponding to OsMADS17 and OsAP2-39 were inserted into the pAbAi vector to generate bait constructs. The bait constructs combined with the pGADT7 empty vector were used as negative controls. The different combinations were co-transformed into yeast strain Y1H Gold. Transformants were grown on the SD/-Leu solid medium at 30 °C for 3 days, and then the transformants were transformed to the SD/-Leu solid medium with or without Aureobasidin A (AbA) at 30 °C for 3–4 days to examine the interactions.
EMSA analysis
The MBP protein, MBP fused protein MBP-OsMADS1 and MBP-OsMADS17 were generated as described in the part of Western blot analysis. The 5′-biotin-labeled DNA fragments containing the binding site were synthesized and used as probes, and unlabeled DNA fragments of the same sequences were used in competitive reactions. Competition for binding was performed with the cold (unlabeled) probe containing the binding site at 20× and 200× the amount of the labeled probe. Each binding reaction in a total reaction volume of 20 μL contained 2 μL biotin-labeled probe, 5 μg protein, 2 μL 10×binding buffer, and 1 μL 50% (v/v) glycerol. The binding reactions were incubated for 30 min at room temperature and then resolved by electrophoresis on 6% (w/v) native polyacrylamide gels in 0.5×TBE buffer. The biotin-labeled probes were detected using a LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific).
Dual-luciferase reporter assay for transactivation analysis
The coding sequences of OsMADS1 and OsMADS17 were inserted into the vector pGreenII 62-SK to form effector constructs, and fragments containing CArG motifs in the regulatory region of OsMADS17 and OsAP2-39 were inserted into the vector pGreenII 0800-LUC to generate reporter constructs56. A CaMV 35S promoter-driven Renilla luciferase (REN) gene was used as an internal control. Constructs were co-transformed (5 μg effector plasmid and 5 μg reporter plasmid) into rice protoplasts for transient expression studies. Co-transfection with the reporter construct and an empty effector construct was used for control. Rice protoplast isolation, plasmid transfection, protoplast incubation, and relative luciferase activities measurement were described earlier in the transient expression assays for translation efficiency analysis.
Natural variation analysis for OsMADS1, OsMADS17, and OsAP2-39
The wild rice accessions used for genotype analysis are listed in Supplementary Data 3. All data on the cultivated rice accessions in the 3K RG for the association test, phenotype evaluation, genotype analysis, and geographical distribution analysis were obtained from the RFGB v2.0 database (https://www.rmbreeding.cn/)60. The frequency of favorable genotypes was analyzed according to the critical variation sites for OsMADS17 (De65-bp/In65-bp), OsAP2-39 (T/C), and OsMADS1 (AS/NS) among 1,760 indica rice, 819 japonica rice, and 196 aus rice accessions. For grain weight analysis, the haplotypes of OsMADS1 in the 3K RG were classified according to the variants with minor allele frequency >0.05 within the transcript region, and the haplotypes of OsAP2-39 were constructed by the variants accepted for the association test. The rare haplotypes present in a total of <20 varieties are not shown among the 1039 indica rice and 562 japonica rice varieties. The geographical distribution of cultivated rice in Asia areas was analyzed by map package (R v4.1.1) using 1507 indica rice and 549 japonica rice varieties. The number of varieties <10 with only one favorable genotype of OsMADS1AS, OsMADS17De65-bp, or OsAP2-39T and <3 with two or three favorable genotypes not shown.
Data analysis
QTL analysis was performed by the Map Manager QTXb2061. Association analysis was performed with Fisher’s exact test, as Fisher’s exact test can be powerful for qualitative traits such as grain weight22. Comparisons are made by two-tailed Student’s t-test and one-way ANOVA with Tukey’s multiple comparisons test (P < 0.05) using SPSS version 17 (SPSS Inc., Chicago, IL, USA), and data were presented as mean ± standard deviation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this Article is available as a Supplementary Information file. Data of genotype, phenotype (1000-grain weight), subgroup classification, and origin for rice accessions are available on the RFGB v2.0 database [https://www.rmbreeding.cn/]. Information on gene sequence and annotation are available on Rice Genome Annotation Project [http://rice.uga.edu/]. Source data are provided with this paper.
References
Jin, J. et al. Genetic control of rice plant architecture under domestication. Nat. Genet. 40, 1365–1369 (2008).
Tan, L. et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 40, 1360–1364 (2008).
Wu, Y. et al. Deletions linked to PROG1 gene participate in plant architecture domestication in Asian and African rice. Nat. Commun. 9, 4157 (2018).
Zhu, Z. et al. Genetic control of inflorescence architecture during rice domestication. Nat. Commun. 4, 2200 (2013).
Ishii, T. et al. OsLG1 regulates a closed panicle trait in domesticated rice. Nat. Genet. 45, 462–465 (2013).
Huang, Y. et al. Variation in the regulatory region of FZP causes increases in secondary inflorescence branching and grain yield in rice domestication. Plant J. 96, 716–733 (2018).
Bai, X. et al. Duplication of an upstream silencer of FZP increases grain yield in rice. Nat. Plants 3, 885–893 (2017).
Gu, B. et al. An-2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Mol. Plant 8, 1635–1650 (2015).
Hua, L. et al. LABA1, a domestication gene associated with long, barbed awns in wild rice. Plant Cell 27, 1875–1888 (2015).
Bessho-Uehara, K. et al. Loss of function at RAE2, a previously unidentifed EPFL, is required for awnlessness in cultivated Asian rice. Proc. Natl Acad. Sci. USA 113, 8969–8974 (2016).
Jin, J. et al. GAD1 encodes a secreted peptide that regulates grain number, grain length, and awn development in rice domestication. Plant Cell 28, 2453–2463 (2016).
Luo, J. et al. An-1 encodes a basic Helix-Loop-Helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 25, 3360–3376 (2013).
Shomura, A. et al. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40, 1023–1028 (2008).
Chen, W. et al. Convergent selection of a WD40 protein that enhances grain yield in maize and rice. Science 375, eabg7985 (2022).
Ashikari, M. et al. Cytokinin oxidase regulates rice grain production. Science 309, 741–745 (2005).
Xue, W. et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 40, 761–767 (2008).
Huang, X. et al. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497 (2009).
Fujita, D. et al. NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proc. Natl Acad. Sci. USA 110, 20431–20436 (2013).
Zhao, L. et al. PAY1 improves plant architecture and enhances grain yield in rice. Plant J. 83, 528–536 (2015).
Jiao, Y. et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544 (2010).
Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549 (2010).
Huo, X. et al. NOG1 increases grain production in rice. Nat. Commun. 8, 1497 (2017).
Wu, X. et al. Enhancing rice grain production by manipulating the naturally evolved cis-regulatory element-containing inverted repeat sequence of OsREM20. Mol. Plant 14, 997–1011 (2021).
Guo, T. et al. ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice. Plant Cell 32, 2763–2779 (2020).
Yin, C., Zhu, Y., Li, X. & Lin, Y. Molecular and genetic aspects of grain number determination in rice (Oryza sativa L.). Int. J. Mol. Sci. 22, 728 (2021).
Song, X. J., Huang, W., Shi, M., Zhu, M. Z. & Lin, H. X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39, 623–630 (2007).
Fan, C. et al. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171 (2006).
Ishimaru, K. et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707–711 (2013).
Sun, L. et al. GS6, a member of the GRAS gene family, negatively regulates grain size in rice. J. Integr. Plant Biol. 55, 938–949 (2013).
Liu, Q. et al. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 9, 852 (2018).
Yu, J. et al. Alternative splicing of OsLG3b controls grain length and yield in japonica rice. Plant Biotechnol. J. 16, 1667–1678 (2018).
Li, Y. et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269 (2011).
Wang, S. et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954 (2012).
Wang, S. et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47, 949–954 (2015).
Wang, Y. et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 47, 944–948 (2015).
Si, L. et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 48, 447–456 (2016).
Ruan, B. et al. Natural variation in the promoter of TGW2 determines grain width and weight in rice. N. Phytol. 227, 629–640 (2020).
Wang, L. OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J. 57, 498–510 (2009).
Shi, C. L. et al. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 103, 1174–1188 (2020).
Tang, Z. et al. Natural variation in OsGASR7 regulates grain length in rice. Plant Biotechnol. J. 19, 14–16 (2021).
Huang, L. et al. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 33, 1212–1228 (2021).
Chen, K., Łyskowski, A., Jaremko, Ł. & Jaremko, M. Genetic and molecular factors determining grain weight in rice. Front. Plant Sci. 12, 605799 (2021).
Yaish, M. W. et al. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 6, e1001098 (2010).
Hu, Y. et al. Interactions of OsMADS1 with floral homeotic genes in rice flower development. Mol. Plant 8, 1366–1384 (2015).
Doebley, J. F., Gaut, B. S. & Smith, B. D. The molecular genetics of crop domestication. Cell 127, 1309–1321 (2006).
Wang, F. et al. The rice circadian clock regulates tiller growth and panicle development through strigolactone signaling and sugar sensing. Plant Cell 32, 3124–3138 (2020).
Zhao, J. et al. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J. Exp. Bot. 70, 6277–6291 (2019).
Sui, Z. et al. Overexpression of peptide-encoding OsCEP6.1 results in pleiotropic effects on growth in rice (O. sativa). Front. Plant Sci. 7, 228 (2016).
Wang, E. et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 40, 1370–1374 (2008).
Liu, Z. et al. OsMKKK70 regulates grain size and leaf angle in rice through the OsMKK4-OsMAPK6-OsWRKY53 signaling pathway. J. Integr. Plant Biol. 63, 2043–2057 (2021).
Buccitelli, C. & Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 21, 630–644 (2020).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001).
Javelle, M., Marco, C. F. & Timmermans, M. In situ hybridization for the precise localization of transcripts in plants. J. Vis. Exp. 57, e3328 (2011).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13 (2005).
Si, X., Zhang, H., Wang, Y., Chen, K. & Gao, C. Manipulating gene translation in plants by CRISPR-Cas9-mediated genome editing of upstream open reading frames. Nat. Protoc. 15, 338–363 (2020).
Yi, X., Du, Z. & Su, Z. PlantGSEA: a gene set enrichment analysis toolkit for plant community. Nucleic Acids Res. 41, W98–W103 (2013).
Tian, T. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129 (2017).
Wang, C. C. et al. Towards a deeper haplotype mining of complex traits in rice with RFGB v2.0. Plant Biotechnol. J. 18, 14–16 (2020).
Manly, K. F., Cudmore, R. J. & Meer, J. M. Map Manager QTX, cross-platform software for genetic mapping. Mamm. Genome 12, 930–932 (2001).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant 31830065 and grant 91935302), the 2115 Talent Development Program of China Agricultural University, and the Science and Technology Innovation Program of Hunan Province (grant 2022RC4036). We thank Professor Zichao Li and Professor Zhanying Zhang for providing the NIL-OsMADSAS and NIL-OsMADSNS materials. We thank Professor Susan R. McCouch for providing the cultivated rice germplasm.
Author information
Authors and Affiliations
Contributions
C.S. and H.S. designed and supervised this study. Y.L. performed experiments and analysis. S.W. provided assistance in collecting the phenotypic data. Y.H. constructed the introgression line 8IL73. X.M. helped analyze the data. F.L., Z.Z., Y.F., K.Z., and P.G. conducted the collection of rice germplasm. Q.L. and M.H. provided technical assistance. Y.L., L.T., D.X., H.S., and C.S. wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiangdong Fu, Hitoshi Mori and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Wu, S., Huang, Y. et al. OsMADS17 simultaneously increases grain number and grain weight in rice. Nat Commun 14, 3098 (2023). https://doi.org/10.1038/s41467-023-38726-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38726-9
This article is cited by
-
RGA1 regulates grain size, rice quality and seed germination in the small and round grain mutant srg5
BMC Plant Biology (2024)
-
Identification of qGL4.1 and qGL4.2, two closely linked QTL controlling grain length in rice
Molecular Breeding (2024)
-
A major QTL simultaneously increases the number of spikelets per spike and thousand-kernel weight in a wheat line
Theoretical and Applied Genetics (2023)
-
qGLF5 from Oryza rufipogon Griff. improves kernel shape, plant architecture, and yield in rice
Theoretical and Applied Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.